Renal Disease and Protease Inhibitors in Aging HIV Patients
 IVRenal disease has been a significant complication of human immunodeficiency virus (HIV) infection since the beginning of the epidemic. However, iatrogenic renal disease produced or exacerbated by antiretroviral (ARV) therapy is a much more recent concern. Initial reports of ARV renal toxicity in patients with otherwise well controlled HIV were initially thought to be uncommon and largely confined to agents within the nucleoside reverse transcriptase inhibitor (NRTI) class, but the potential for renal toxicity from protease inhibitors (PIs) has been a growing clinical issue, particularly in the context of the aging HIV population in which declining renal function has become common. There is now a large body of data indicating that relative risk of renal impairment from PIs is not evenly distributed across this drug class. Understanding the relative risk of renal impairment with PIs may help in therapy selection.
IVRenal disease has been a significant complication of human immunodeficiency virus (HIV) infection since the beginning of the epidemic. However, iatrogenic renal disease produced or exacerbated by antiretroviral (ARV) therapy is a much more recent concern. Initial reports of ARV renal toxicity in patients with otherwise well controlled HIV were initially thought to be uncommon and largely confined to agents within the nucleoside reverse transcriptase inhibitor (NRTI) class, but the potential for renal toxicity from protease inhibitors (PIs) has been a growing clinical issue, particularly in the context of the aging HIV population in which declining renal function has become common. There is now a large body of data indicating that relative risk of renal impairment from PIs is not evenly distributed across this drug class. Understanding the relative risk of renal impairment with PIs may help in therapy selection.
Jordan Weinstein, MD | Mélanie Hamel, MD Clinique du Quartier Latin Clinique l’Actuel, Montréal, Québec Division of Nephrology Hôpital Cité de la Santé University of Montréal, Laval, Québec | Joss de Wet, MD, MBChB, CCFP |
 This educational resource has been reviewed by the Canadian Society of Nephrology and it supports the use of this reference in an individual's claim under Section 2: Self-Learning credits for time spent in this activity in the MOC program as defined by the Royal College of Physicians and Surgeons of Canada's Maintenance of Certification program. Members of The College of Family Physicians of Canada may claim Mainpro-M2 credits for time spent in this unaccredited educational activity. Members may also complete a practice reflection exercise and generate Mainpro-C credits. For more information, please refer to "Linking Learning to Practice" from www.cfpc.ca. The information provided in this program does not necessarily reflect the opinions and recommendations of the Canadian Society of Nephrology. Any products mentioned should be used in accordance with the prescribing information contained in their respective Product Monographs. This educational resource has been reviewed by the Canadian Society of Nephrology and it supports the use of this reference in an individual's claim under Section 2: Self-Learning credits for time spent in this activity in the MOC program as defined by the Royal College of Physicians and Surgeons of Canada's Maintenance of Certification program. Members of The College of Family Physicians of Canada may claim Mainpro-M2 credits for time spent in this unaccredited educational activity. Members may also complete a practice reflection exercise and generate Mainpro-C credits. For more information, please refer to "Linking Learning to Practice" from www.cfpc.ca. The information provided in this program does not necessarily reflect the opinions and recommendations of the Canadian Society of Nephrology. Any products mentioned should be used in accordance with the prescribing information contained in their respective Product Monographs. |
Background
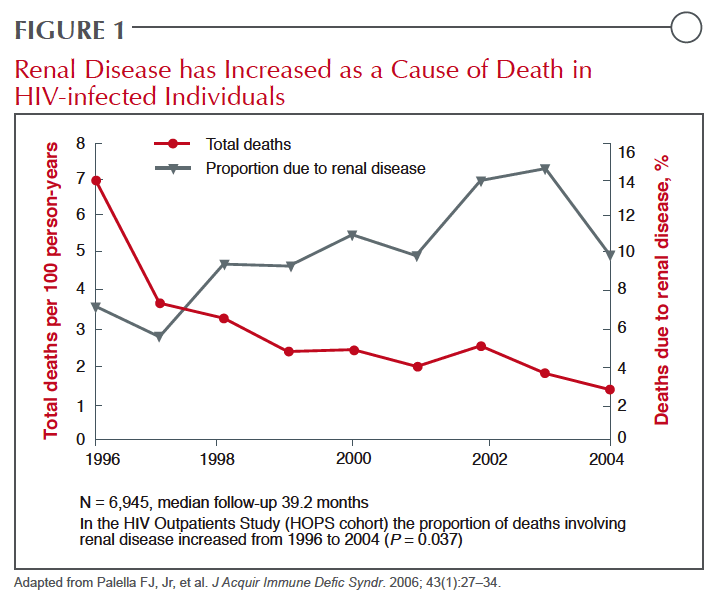 Human immunodeficiency virus associated nephrotoxicity (HIVAN) was a prominent cause of HIV-related death prior to the introduction of treatment regimens capable of viral suppression.1 Since the introduction of protease inhibitors (PIs), which were instrumental within the first regimens to permit long-term survival,2 HIVAN has become relatively rare, particularly in those HIV patients who initiate therapy before severe immunologic depletion.3 The current evidence associating selected PIs with accelerated renal impairment suggests that this therapy has largely eliminated one complication to potentially create another. While no therapeutic goal in the management of HIV takes precedence over sustained viral suppression, the growing proportion of HIV-infected individuals in Canada and elsewhere who have reached or passed middle-age is altering relative treatment risks. Renal impairment, along with cardiovascular (CV) disease, osteoporosis, and cognitive decline, are among progressive conditions that now threaten the ability of HIV patients with otherwise well controlled infection to expect a normal lifespan.4 After a steep decline in kidney-related deaths with the introduction of effective HIV therapies, mortality due to this cause has been rising (Figure 1). Although deaths directly attributable to renal failure may be plateauing in Canada and other Western countries where renal transplant is available in patients with HIV, it is clear that renal disease unrelated to HIVAN is increasing.
Human immunodeficiency virus associated nephrotoxicity (HIVAN) was a prominent cause of HIV-related death prior to the introduction of treatment regimens capable of viral suppression.1 Since the introduction of protease inhibitors (PIs), which were instrumental within the first regimens to permit long-term survival,2 HIVAN has become relatively rare, particularly in those HIV patients who initiate therapy before severe immunologic depletion.3 The current evidence associating selected PIs with accelerated renal impairment suggests that this therapy has largely eliminated one complication to potentially create another. While no therapeutic goal in the management of HIV takes precedence over sustained viral suppression, the growing proportion of HIV-infected individuals in Canada and elsewhere who have reached or passed middle-age is altering relative treatment risks. Renal impairment, along with cardiovascular (CV) disease, osteoporosis, and cognitive decline, are among progressive conditions that now threaten the ability of HIV patients with otherwise well controlled infection to expect a normal lifespan.4 After a steep decline in kidney-related deaths with the introduction of effective HIV therapies, mortality due to this cause has been rising (Figure 1). Although deaths directly attributable to renal failure may be plateauing in Canada and other Western countries where renal transplant is available in patients with HIV, it is clear that renal disease unrelated to HIVAN is increasing.
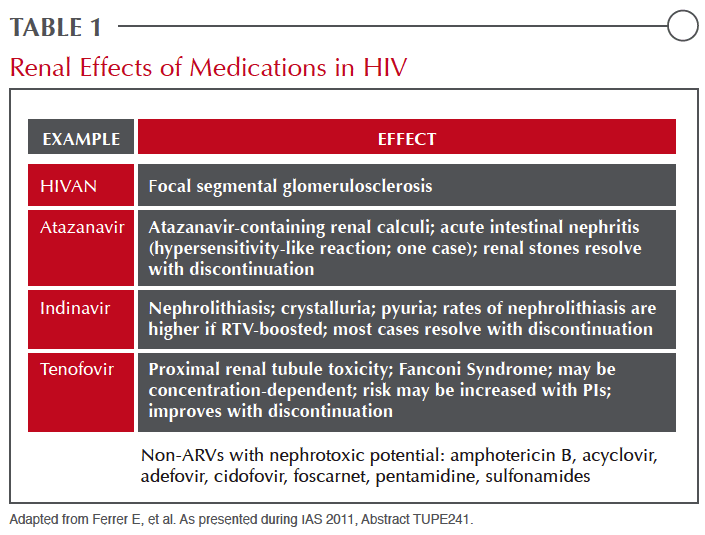 While HIV-infected individuals share the same risks for chronic kidney disease (CKD), such as diabetic nephropathy or hypertension, that occur in those without HIV, the causes and types of renal impairment and nephrotoxicity unique to this population vary substantially. HIVAN is an acute process characterized by focal segmental glomerulosclerosis caused by HIV activity in the kidney and is largely confined to Blacks in the era of effective antiretroviral (ARV) therapy. The nephrotoxicity most closely associated with nucleoside reverse transcriptase inhibitors (NRTIs) such as tenofovir (TDF) is attributed to proximal renal tube toxicity.5 This same type of injury may be caused by a variety of non-ARV therapies, particularly antimicrobials such as gentamicin and amphotericin B.6 TDF may also cause Fanconi syndrome, which is far less common, but Fanconi syndrome is not a prerequisite for major renal injury from this agent. PIs are most closely associated with crystalluria and tubule obstruction as well as acute interstitial nephritis.7 While all of these can lead to renal injury as measured by estimated glomerular filtration rate (eGFR) or the presence of proteinuria, the differences in pathophysiology are clinically relevant (Table 1).8
While HIV-infected individuals share the same risks for chronic kidney disease (CKD), such as diabetic nephropathy or hypertension, that occur in those without HIV, the causes and types of renal impairment and nephrotoxicity unique to this population vary substantially. HIVAN is an acute process characterized by focal segmental glomerulosclerosis caused by HIV activity in the kidney and is largely confined to Blacks in the era of effective antiretroviral (ARV) therapy. The nephrotoxicity most closely associated with nucleoside reverse transcriptase inhibitors (NRTIs) such as tenofovir (TDF) is attributed to proximal renal tube toxicity.5 This same type of injury may be caused by a variety of non-ARV therapies, particularly antimicrobials such as gentamicin and amphotericin B.6 TDF may also cause Fanconi syndrome, which is far less common, but Fanconi syndrome is not a prerequisite for major renal injury from this agent. PIs are most closely associated with crystalluria and tubule obstruction as well as acute interstitial nephritis.7 While all of these can lead to renal injury as measured by estimated glomerular filtration rate (eGFR) or the presence of proteinuria, the differences in pathophysiology are clinically relevant (Table 1).8
The specific risks of each of these complications has been derived largely from case-control studies, and there has been considerable variability in reported risk, possibly due to relative differences in the susceptibility of study populations for renal disease, which can be influenced by the age of the patient, the history of HIV control, and exposure to other therapies that pose a risk of nephrotoxicity.6 In sum, the risk for nephrotoxicity from ARVs overall and PIs specifically appears to be low in a young, otherwise healthy population with optimal HIV suppression.9 Risk of renal function decline increases in age in the HIV population as it does in the non-HIV population10-11, although this risk in patients with HIV appears to be accelerated, particularly in patients exposed to nephrotoxic therapies.12
Epidemiology
 The prevalence of renal impairment in patients with HIV is dependent on the definition. While up to 30% of HIV patients in an outpatient setting have demonstrated proteinuria in some series,13 the rate of impaired kidney function as traditionally defined by an eGFR <60 mL/min per 1.73 m2, is considerably less, ranging from 2.5% to 10% in published studies.14-15 While these figures are highly dependent on the median age of patient samples as well as the prevalence of risk factors within the populations studied, it has been estimated that approximately 15% of proximal renal tubular dysfunction (PRTD) is due to ARV therapy.16 However, the contribution of ARVs may be higher in highly-susceptible patients, such as those with pre-existing renal impairment.
The prevalence of renal impairment in patients with HIV is dependent on the definition. While up to 30% of HIV patients in an outpatient setting have demonstrated proteinuria in some series,13 the rate of impaired kidney function as traditionally defined by an eGFR <60 mL/min per 1.73 m2, is considerably less, ranging from 2.5% to 10% in published studies.14-15 While these figures are highly dependent on the median age of patient samples as well as the prevalence of risk factors within the populations studied, it has been estimated that approximately 15% of proximal renal tubular dysfunction (PRTD) is due to ARV therapy.16 However, the contribution of ARVs may be higher in highly-susceptible patients, such as those with pre-existing renal impairment.
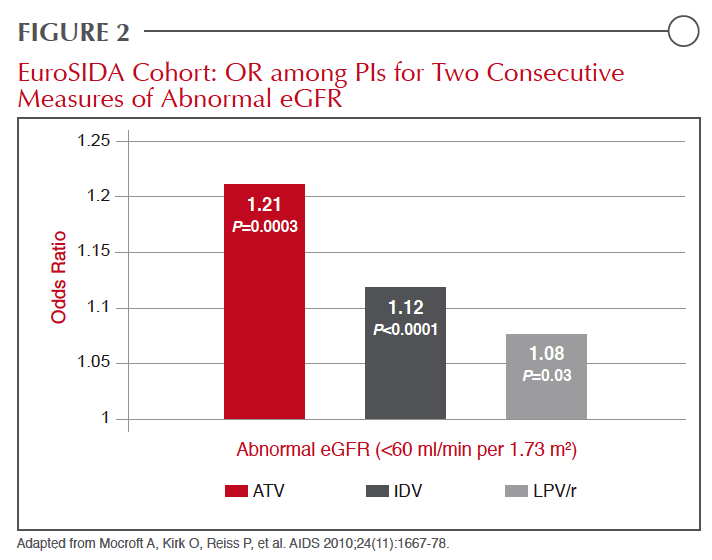
The interest in the relative role of different PIs in causing or exacerbating renal impairment has generated a variety of efforts to gauge risk. One of the largest involved an analysis of the EuroSIDA cohort. In that population, the odds ratio (OR) among PIs for two consecutive measures of abnormal eGFR (<60 ml/min per 1.73 m2) were 1.21 for atazanavir (ATV) (P=0.0003), 1.12 for indinavir (IDV) (P<0.0001), and 1.08 for lopinavir (LPV)/r (P=0.03) Figure 2.5 In the more recently published cross- sectional French Aquitaine study, the goal was to evaluate the incidence of PRTD. In this set of data, ATV was associated with a slightly greater risk of renal dysfunction (OR 1.28 per year of exposure) than TDF (OR 1.23 per year of exposure).17 No other PI was associated with a significant risk of PRTD on multivariate analysis, although ritonavir has long been associated with nephrotoxicity and increased risk of renal failure.18 Referring to the cumulative risk of AKI with continued exposure, the authors of that study expressed concern about reversibility for those maintained on therapy once renal function declines.
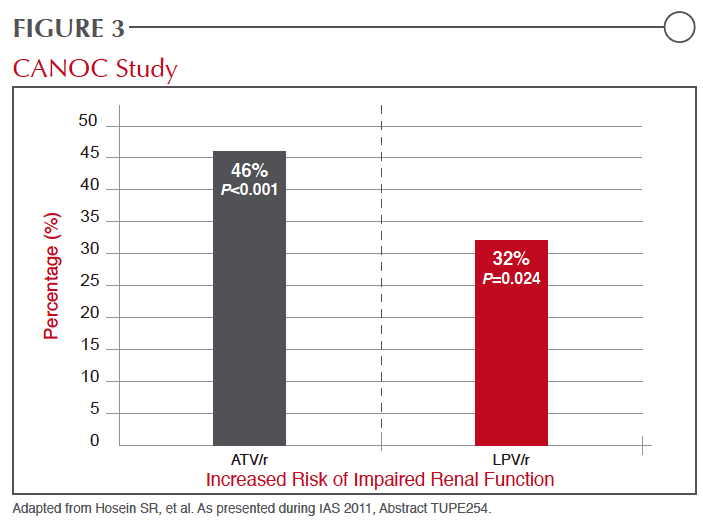 In a controlled study of 322 treatment-naive patients randomized to initiate therapy with ATV/r or the non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz (EFV), both combined with TDF plus emtricitabine (FTC), eGFR was significantly reduced in the ATV/r arm at 48 weeks.19 Presented at the 2011 Conference on Retroviruses and Opportunistic Infections (CROI), there was no interaction for change in renal function and ethnicity, even though Blacks were more likely than Caucasians to have baseline abnormalities, but age was identified as a predictor of increased likelihood of renal function decline over the course of the study. At the recent 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention (IAS 2011), data from the Canadian Observational Cohort Collaboration (CANOC) was presented on two PIs, ATV and LPV.(20) On the basis of data generated by 985 patients being followed at eight collaborating centres in British Columbia, Ontario and Quebec, the hazard ratio (HR) for impaired kidney function by the traditional eGFR definition, was increased 46% (HR 1.46; P<0.001) for ATV and 32% (HR 1.32; P=0.024) for LPV when both were compared to ARV exposures that did not include a PI (Figure 3).
In a controlled study of 322 treatment-naive patients randomized to initiate therapy with ATV/r or the non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz (EFV), both combined with TDF plus emtricitabine (FTC), eGFR was significantly reduced in the ATV/r arm at 48 weeks.19 Presented at the 2011 Conference on Retroviruses and Opportunistic Infections (CROI), there was no interaction for change in renal function and ethnicity, even though Blacks were more likely than Caucasians to have baseline abnormalities, but age was identified as a predictor of increased likelihood of renal function decline over the course of the study. At the recent 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention (IAS 2011), data from the Canadian Observational Cohort Collaboration (CANOC) was presented on two PIs, ATV and LPV.(20) On the basis of data generated by 985 patients being followed at eight collaborating centres in British Columbia, Ontario and Quebec, the hazard ratio (HR) for impaired kidney function by the traditional eGFR definition, was increased 46% (HR 1.46; P<0.001) for ATV and 32% (HR 1.32; P=0.024) for LPV when both were compared to ARV exposures that did not include a PI (Figure 3).
A second study presented at this year's IAS meeting from a UK database showed similar relative differences. In this review of electronic medical records from 2,115 patients starting an ARV regimen, all of whom had an eGFR >60 ml/min per 1.732 at baseline, risk was calculated after adjusting for gender, age, baseline eGFR, baseline CD4 count, hepatitis B and C status, and prior exposure to TDF and IDV.(21) Compared to EFV, which served as a reference, the increase in risk did not reach significance for darunavir (DRV) (P=0.108), reached modest significance for LPV (P=0.017), and was most strongly significant for ATV (P=0.004). Overall, these data confirm disparate risks for renal risk among PIs.
Mechanism of PI-Induced Renal Impairment
 While PRTD, which is the major mechanism of nephrotoxicity induced by TDF, has been associated with PIs, the more prominent mechanisms of PI-induced acute renal impairment are acute interstitial nephritis (AIN) and crystalluria.6 While AIN is allergic-like reaction in which an elevated serum creatinine is often accompanied by such symptoms as rash, fever, and peripheral eosinophilia, only a proportion of individuals have the classic clinical features, so that either kidney biopsy or drug discontinuation is frequently needed to confirm the diagnosis.6 In such cases, a mechanism of injury other than AIN is possible. These may also be reversible by drug discontinuation. A variety of non-ARV drugs have been associated with AIN, but IDV, the first PI associated with the complication,22 remains one of the most closely associated. Reports of AIN with ATV began appearing soon after the introduction of this agent,21-22 while other PIs, based on the low number of reports, appear to pose a lower risk for this complication.
While PRTD, which is the major mechanism of nephrotoxicity induced by TDF, has been associated with PIs, the more prominent mechanisms of PI-induced acute renal impairment are acute interstitial nephritis (AIN) and crystalluria.6 While AIN is allergic-like reaction in which an elevated serum creatinine is often accompanied by such symptoms as rash, fever, and peripheral eosinophilia, only a proportion of individuals have the classic clinical features, so that either kidney biopsy or drug discontinuation is frequently needed to confirm the diagnosis.6 In such cases, a mechanism of injury other than AIN is possible. These may also be reversible by drug discontinuation. A variety of non-ARV drugs have been associated with AIN, but IDV, the first PI associated with the complication,22 remains one of the most closely associated. Reports of AIN with ATV began appearing soon after the introduction of this agent,21-22 while other PIs, based on the low number of reports, appear to pose a lower risk for this complication.
Crystalluria, which can also precipitate renal injury, describes drug crystals precipitated in the renal tubule lumen due to insolubility in the urine. Again, IDV was the first PI with which this form of nephrotoxicity was associated,25 but other agents, particularly ATV, have subsequently been linked to this complication.27 In addition to impaired renal function, crystalluria can lead to nephrolithiasis as well as more insidious forms of renal injury.26 In data generated from a survey of a UK database, which were presented at the 2011 IAS meeting, a substantially increased risk of kidney stones was associated with ATV relative to DRV, LPV, or EFV.(28) While the rate of stones was similar for LPV, EFV, and DRV, the rate of stones on ATV was 7.3 per 1000 patient years of exposure or more than three times higher (P<0.001) even though the absolute risk in all groups remains low. The relative increase in risk remained similar after excluding patients previously exposed to IDV Table 2.
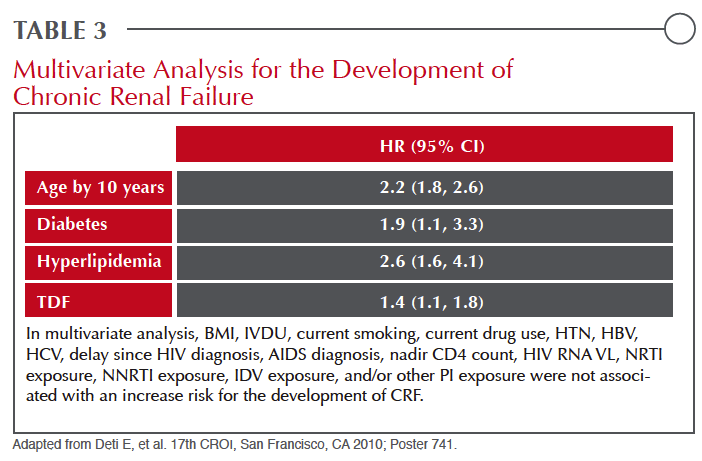 While PIs appear to differ markedly for the risk of nephrotoxicity, individual risk factors are important. Therisk of crystal nephropathy, for example, is increased for all drugs capable of inducing this complication, not just PIs, with volume depletion and diminished urine flow.6 Similar to patients without HIV, the HR for chronic renal failure as defined by eGFR more than doubles with each decade of age, more than doubles in the presence of hyperlipidemia, and nearly doubles in the presence of diabetes Table 3.29 Primary hypertension is one of the leading causes of nephropathy in aging individuals even in the absence of HIV.30 Genetic predisposition for renal complications is also likely to be important for individual risk assessment, possibly providing at least part of the explanation for the far greater risk for renal complications among Blacks with HIV,3 which is linked to missense mutations in the APOL1 gene.31, 32
While PIs appear to differ markedly for the risk of nephrotoxicity, individual risk factors are important. Therisk of crystal nephropathy, for example, is increased for all drugs capable of inducing this complication, not just PIs, with volume depletion and diminished urine flow.6 Similar to patients without HIV, the HR for chronic renal failure as defined by eGFR more than doubles with each decade of age, more than doubles in the presence of hyperlipidemia, and nearly doubles in the presence of diabetes Table 3.29 Primary hypertension is one of the leading causes of nephropathy in aging individuals even in the absence of HIV.30 Genetic predisposition for renal complications is also likely to be important for individual risk assessment, possibly providing at least part of the explanation for the far greater risk for renal complications among Blacks with HIV,3 which is linked to missense mutations in the APOL1 gene.31, 32
Managing Renal Risk in Patients on PIs
Identifying and minimizing renal impairment in patients with HIV should now be considered a routine part of patient care. Renal disease remains an important comorbidity in patients with HIV, including those whose infection is controlled. Moreover, renal disease is an important independent predictor of CV events. Both declining eGFR and proteinuria correlated with increasing risk after controlling for other CV risk factors.33
There are several approaches for monitoring renal function in HIV patients. Serial measurements of proteinuria are useful for identifying both glomerular and renal tubular injuries, but serum creatinine and eGFR are more predictive measures of kidney function. While comprehensive studies of renal function are important at the initiation of therapy in order to provide baseline values to monitor change, the frequency with which subsequent measures are taken should be driven by risk factors for renal disease, such as diabetes, the age of the patient, and the relative risk of nephrotoxicity for both the ARV agents and other drugs with nephrotoxic potential that the patient is taking.
The management of HIV frequently requires a balance of risks and benefits. Renal impairment is an important consideration, but it cannot be considered in isolation.
Control of viremia is an essential first step. The risk of nephrotoxicity with certain medications should not be minimized, particularly in young patients, patients without other significant risks for renal impairment, or black patients. However, in an aging population who are candidates for PIs, selecting an agent with a low relative risk may be prudent in the context of other clinical issues.
Conclusion
Driven in part by the aging of HIV patients in Canada, as in many other industrialized countries, the risks of age-related organ dysfunction are being given more intensive scrutiny. The evidence that patients with HIV develop age-related diseases at an accelerated pace has produced a new threat to the goal of providing infected patients with a normal lifespan. Renal disease is an insidious process that often does not produce symptoms until dysfunction has reached relatively advanced stages. While many factors affect risk of renal disease, some PIs appear to pose a greater risk of causing or exacerbating renal impairment than others. Sensitivity to the relative risk of these agents is important in the context of patients rendered susceptible to renal disease by other factors or simply by age.
Summary Points
Renal disease, a function of age in patients without HIV infection, appears to be developing earlier in patients with HIV, a phenomenon that is likely due to specific HIV damage to the kidney, a generalized acceleration of aging across all organs, and specific renal toxicity associated with ARV therapies.
While sustained suppression of HIV is the most important goal in the management of infected individuals, strategies to minimize risks of potentially terminal pathological process, including renal impairment, are increasingly important in aging individuals. While PIs may be part of an NRTI-sparing regimen specifically developed to reduce risks of renal impairment, PI selection appears to be important for benefit-to-risk ratio optimization.
Questions
The phenomenon of accelerated aging in HIV patients has complicated the management of patients whose HIV is otherwise well controlled. Clinicians need to consider the effects of ARV treatments on a spectrum of organs, including the CV system, the liver, the skeleton, the central nervous system, and the kidney. Considering renal risk among PIs is one of the emerging clinical issues. We asked Dr. Joss de Wet, MBChB, CCFP, Clinical Assistant Professor, Department of Family and Community Medicine, University of British Columbia, to develop questions most important to the management of HIV patients, and we posed them to our invited experts.
1. Are there published guidelines that outline the types and intervals for monitoring renal function in patients with HIV and, if so, is there good consensus among experts about these guidelines particularly in regard to how they apply to patients with increased risk of renal disease?
There are no evidence-based guidelines endorsed by a governmental body, but the European AIDS Clinical Society (EACS) has issued guidelines for non-infectious co-morbidities that include a section on diagnosis, prevention, and treatment of kidney disease (http://www.europeanaidsclinicalsociety.org/images/stories/ EACS-Pdf/2_non_infectious_co_morbidities_in_hiv.pdf, at time of printing). There have also been several expert review papers from which some reasonable guidance might be derived, such as the recently published paper by Phair J, et al. (Curr Opin HIV AIDS 2011;6(4):285-9). In general, an initial work-up in a newly diagnosed patient with HIV should include the battery of tests that would be considered in a comprehensive physical. From the renal perspective, this would include a renal function test. While it might be argued that urinary protein excretion measures might be sufficient in an otherwise healthy HIV patient under the age of 30, a baseline measurement of the estimated glomerular filtration rate (eGFR) is prudent. In the absence of risk factors for renal dysfunction, this test can be performed at the initiation of highly active antiretroviral therapy (HAART) and repeated at annual intervals, although it is reasonable to test kidney function with eGFR and urinary protein excretion at more frequent intervals in HIV patients who reach middle age whether or not they have risk factors for renal disease.
2. In HIV patients who develop evidence of a modest decrease in renal function, such as slowly increasing proteinuria, while taking an otherwise effective ARV regimen, how aggressive should one be in changing drugs within the regimen to eliminate those associated with renal impairment? Is each case unique?
Each case is unique, but the most important step is to investigate the cause of GFR diminution or the increase in proteinuria to determine whether the change in renal function is due to the ARV regimen. This is generally achieved by a process of elimination in which the HIV therapies are presumed to be the cause of diminished renal function in the absence of other identifiable causes. There is no formula for switching therapies if ARV treatment is considered to be the underlying problem. It becomes particularly difficult to consider switching strategies in patients who have already developed resistance or intolerance to several ARV therapies, thereby narrowing choices. In long-term treatment of HIV, the single most important principle is providing persistent HIV control. In cases where a broad array of agents are predicted to be effective, evidence of progressive renal impairment should certainly prompt consideration of designing a regimen that eliminates agents with potential to exacerbate renal decline, such as TDF or ATV. In patients with advanced disease, the degree or speed or advancing renal dysfunction should have a major influence on the decision to switch therapies. Although renal transplant is a last resort, it is important to keep in mind that there are more options for controlling end-stage renal disease and HIV when ARV choices have been exhausted.
3. In a patient initiating HIV therapy with risk factors for renal impairment, such as difficult to control hypertension or diabetes, is it appropriate to avoid drugs within a class that are more closely associated with renal impairment, such as TDF among NRTIs or ATV among PIs, or should other concerns take precedence, switching only if renal function changes?
In patients with poor renal function or risk factors for renal disease, such as diabetes, it may be appropriate to avoid drugs associated with renal dysfunction at the outset. The risk of renal impairment from any ARV therapy is relatively low even on long-term therapy. The lifetime risk of CKD on TDF is highly dependent on risk factors but the incidence remains low over the first year of therapy. Cumulative exposure also appears to be important to other agents with the potential for nephrotoxicity, such as ATV. However, for those who already have renal dysfunction, there is reasonable concern that drugs with nephrotoxic potential will accelerate renal damage, particularly over long-term treatment. There are good alternatives in the same drug class to TDF and to ATV, so there is no reason not to consider regimens that will spare adverse effects on the kidney.
4. In a patient with signs of renal impairment on an ARV regimen that includes drugs with potential renal toxicity, are there reasonable alternatives to switching therapy, such as adding an inhibitor of the renin angiotensin system?
In patients with renal impairment, whether or not they are infected with HIV, a RAS inhibitor is considered a standard therapy. However, the use of an ACE inhibitor or an angiotensin receptor blocker cannot be expected to offer adequate protection from renal dysfunction imposed by a nephrotoxic agent, so switching treatments should be considered when alternative agents are available. In patients with renal dysfunction, RAS inhibitors slow the rate of decline rather than treat or reverse the damage. In patients with declining renal function, lifestyle strategies, such as weight loss, smoking cessation, and low-salt diets, should be considered, but due to the progressive nature of renal disease, the effort to prevent further insult is important. In patients with renal impairment, a RAS inhibitor may modify progression but this would not be expected to prevent damage.
5. Is there a threshold of renal dysfunction at which it is appropriate to involve a nephrologist in patient care or is this a decision that should be based on multiple factors, such as the medical history and likelihood of accelerated renal dysfunction?
The guidelines of the Canadian Society of Nephrology recommend referral of all adult patients with or without HIV to a nephrologist when there is a persistent eGFR <30 mL/min x 1.73 m2, progressive decline of kidney function, ratio of urine to protein to creatinine >100 mg/mmol, urine albumin to creatinine ratio >60 mg/mmol, inability to reach treatment targets, or rapid changes in kidney function (Levin A, et al. CMAJ 2008;179(11):1154-62). Patients with less significant renal impairment or proteinuria may not require specialist care if the course appears to be slow and uncomplicated. However, patients with multiple risk factors for renal disease and diminishing function may benefit from specialists who provide a comprehensive screen of contributing risk factors and may help provide guidance on a long-term strategy. It is important to recognize that CV events represent one of the greatest risks of advancing renal disease. Whether or not patients are evaluated by a nephrologist, clinicians should consider renal disease an important signal of increased CV risk and consider evaluating and addressing the full spectrum of factors that contribute to CV events in addition to their effort to control declining renal function.
References
1. Winston JA, Klotman PE. Are we missing an epidemic of HIV-associated nephropathy? J Am Soc Nephrol 1996;7(1):1-7.
2. Wensing AM, van Maarseveen NM, Nijhuis M. Fifteen years of HIV Protease Inhibitors: raising the barrier to resistance. Antiviral Res 2010;85(1):59-74.
3. Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV- infected individuals: a tale of two races. J Infect Dis 2008;197(11):1548-57.
4. Patel D, Crane LR. Growing Old with HIV. Curr Infect Dis Rep 2011;13(1):75-82.
5. Mocroft A, Kirk O, Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS 2010;24(11):1667-78.
6. Fine DM, Perazella MA, Lucas GM, Atta MG. Renal disease in patients with HIV infection: epidemiology, pathogenesis and management. Drugs 2008;68(7):963-80.
7. Anderson PL, Lichtenstein KA, Gerig NE, Kiser JJ, Bushman LR. Atazanavir-containing renal calculi in an HIV-infected patient. AIDS 2007;21(8):1060-2.
8. Jao J, Wyatt CM. Antiretroviral medications: adverse effects on the kidney. Adv Chronic Kidney Dis 2010;17(1):72-82.
9. Brennan A, Evans D, Maskew M, et al. Relationship between renal dysfunction, nephrotoxicity and death among HIV adults on tenofovir. AIDS 2011;25(13):1603-9.
10. Hallan SI, Dahl K, Oien CM, et al. Screening strategies for chronic kidney disease in the general population: follow-up of cross sectional health survey. BMJ 2006;333(7577):1047.
11. Colson AW, Florence E, Augustijn H, Verpooten GA, Lynen L, Gheuens E. Prevalence of chronic renal failure stage 3 or more in HIV-infected patients in Antwerp: an observational study. Acta Clin Belg 2010;65(6):392-8.
12. Campbell LJ, Ibrahim F, Fisher M, Holt SG, Hendry BM, Post FA. Spectrum of chronic kidney disease in HIV-infected patients. HIV Med 2009;10(6):329-36.
13. Szczech LA, Grunfeld C, Scherzer R, et al. Microalbuminuria in HIV infection. AIDS 2007;21(8):1003-9.
14. Fernando SK, Finkelstein FO, Moore BA, Weissman S. Prevalence of chronic kidney disease in an urban HIV infected population. Am J Med Sci 2008;335(2):89-94.
15. Jones CY, Jones CA, Wilson IB, et al. Cystatin C and creatinine in an HIV cohort: the nutrition for healthy living study. Am J Kidney Dis 2008;51(6):914-24.
16. Franceschini N, Napravnik S, Eron JJ, Jr., Szczech LA, Finn WF. Incidence and etiology of acute renal failure among ambulatory HIV-infected patients. Kidney Int 2005;67(4):1526-31.
17. Dauchy FA, Lawson-Ayayi S, de La Faille R, et al. Increased risk of abnormal proximal renal tubular function with HIV infection and antiretroviral therapy. Kidney Int 2011;80(3):302-9.
18. Deray G, Bochet M, Katlama C, Bricaire F. Nephrotoxicity of ritonavir. Presse Med 1998;27(35):1801-3.
19. Dazo C, Fahey P, Puls R, Winston A. Small but significant and non- progressive decline in GFR observed in therapy-naive HIV+ subjects commencing r/ATV compared to either EFV or ZDV/BC with TDF/FTC after 48 weeks: a randomized controlled study. In: Conference on Retroviruses and Opportunistic Infections (CROI); 2011; Boston; 2011.
20. Hosein SR, Johns K, Chan K, Rabound J, Harris M. Progression to impaired estimated golmerular filtration rate (eGFR) between antiretroviral therapy regimens in the Canadian Observational Cohort (CANOC) collaboration. In: International AIDS Society; 2011; Rome, Italy; 2011. p. TUPE254.
21. Rockwood N, Nelson MR, Mandalia S, Gazzard B. A comparative analysis of risk factors associ
