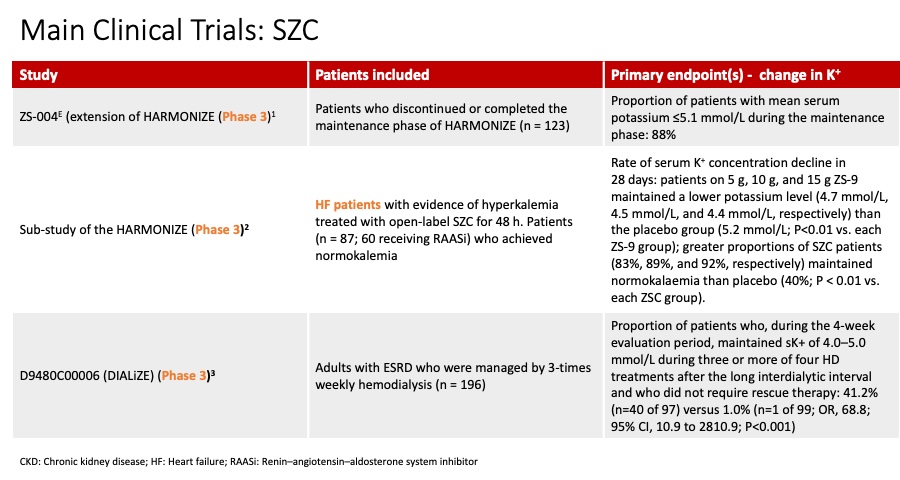
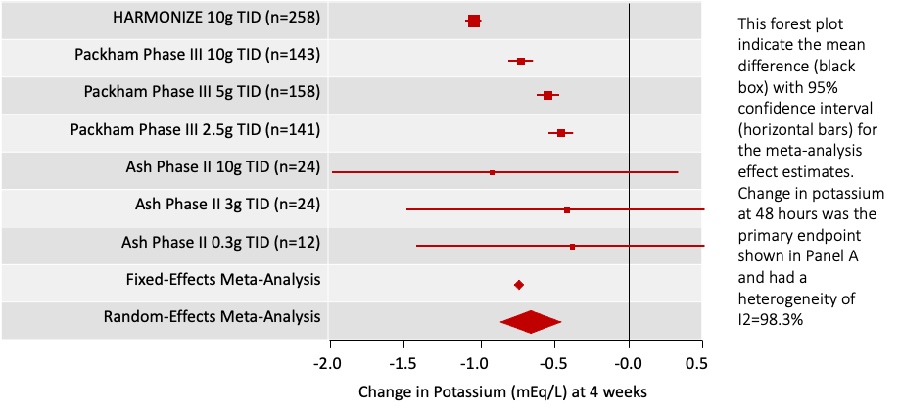
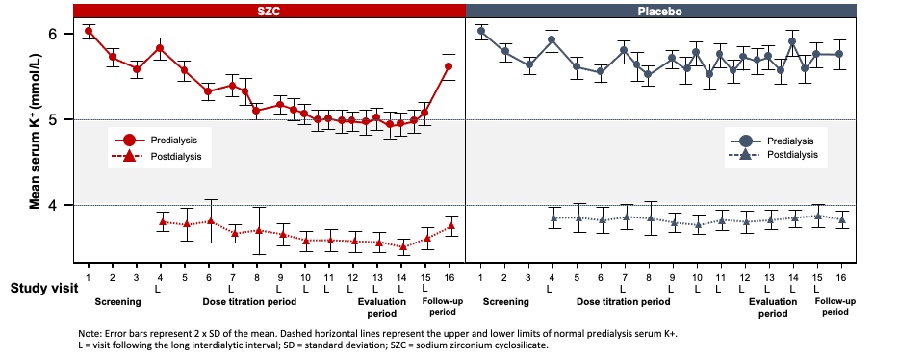
These questions belong to Part 1 of our feature on Hot Topics in Hyperkalemia. Please find Part 2 at the following link
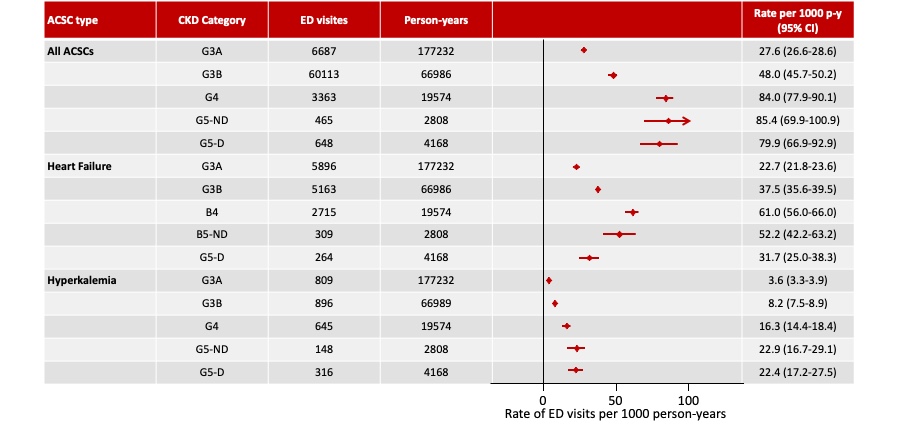
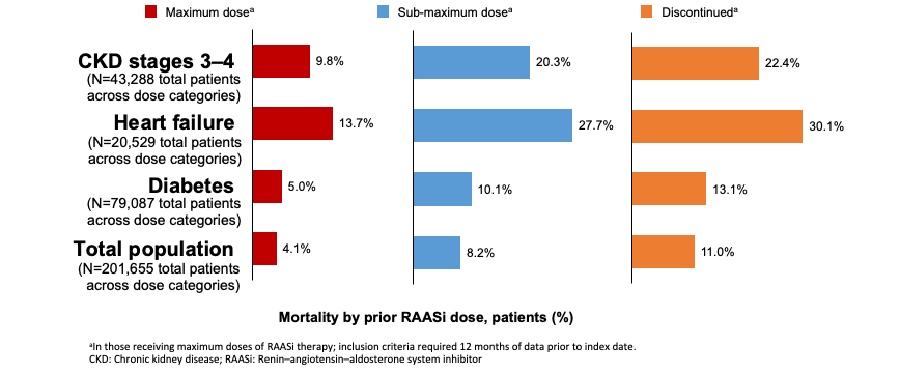
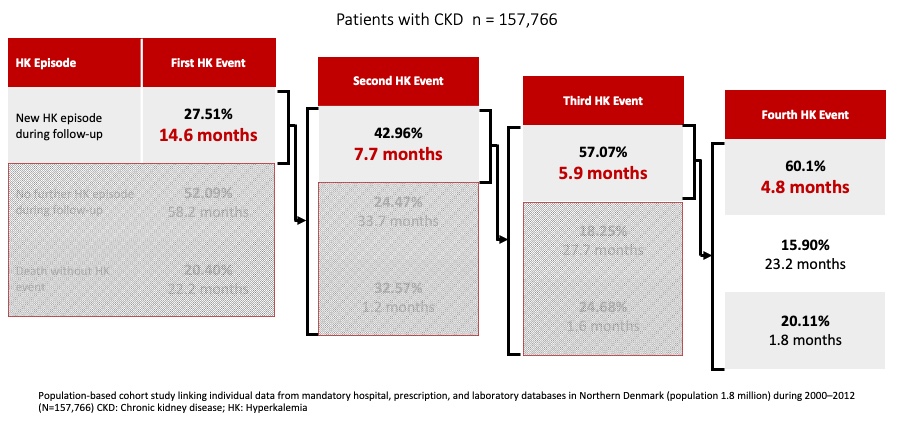
Patients with CKD and HF tend to have recurrent hyperkalemia episodes with successively shorter time between each subsequent episode. In a large population cohort study (n = 157,766) conducted in Denmark between 2000 and 2012, it was shown that among people with CKD that had a first HK event, 42.96% had a second event, with a median time between events of 7.7 months. Patient proportions who experienced a third (57.07%) or fourth (60.10%) hyperkalemia event after previous events were even greater. Similar observations were made among HF patients1[Thomsen 2018, p. 1614/fig.2].
Reference:
- Thomsen RW, Nicolaisen SK, Hasvold P, et al. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes-a Danish population-based cohort study. Nephrol Dial Transplant. 2018 Sep 1;33(9):1610-1620. doi: 10.1093/ndt/gfx312.