UKidney Nephrology News and Insights
Canagliflozin in patients with very low GFR: Protection persists
Excitement for the SGLT2 inhibitor class continued at the 2019 ASN Kidney Week. Evidence continues to accumulate from analyses of the CREDENCE trial which originally showed - for the first time in a randomized controlled study with hard renal outcomes - that patients taking canagliflozin experienced slower decline in their kdiney function and a reduction in the incidence of end-stage renal failure requiring dialysis or kidney transplant. In the CREDENCE study, patients with an estimated eGFR below 30 ml/min were excluded from the trial. However, patients who had a eGFR > 30 ml/min at the time of screening but in whom the eGFR fell below 30 ml/min by the time of randomization were nevertheless included in the study.
Dr. George Bakris presented data in the patients with eGFR less than 30 mil/min at the ASN in Washington. The difference in final eGFR as well as the slope of eGFR decline favored the Canagliflozin group in much the same way as patients in the trial overall. No new safety concerns were identified in this subgroup.
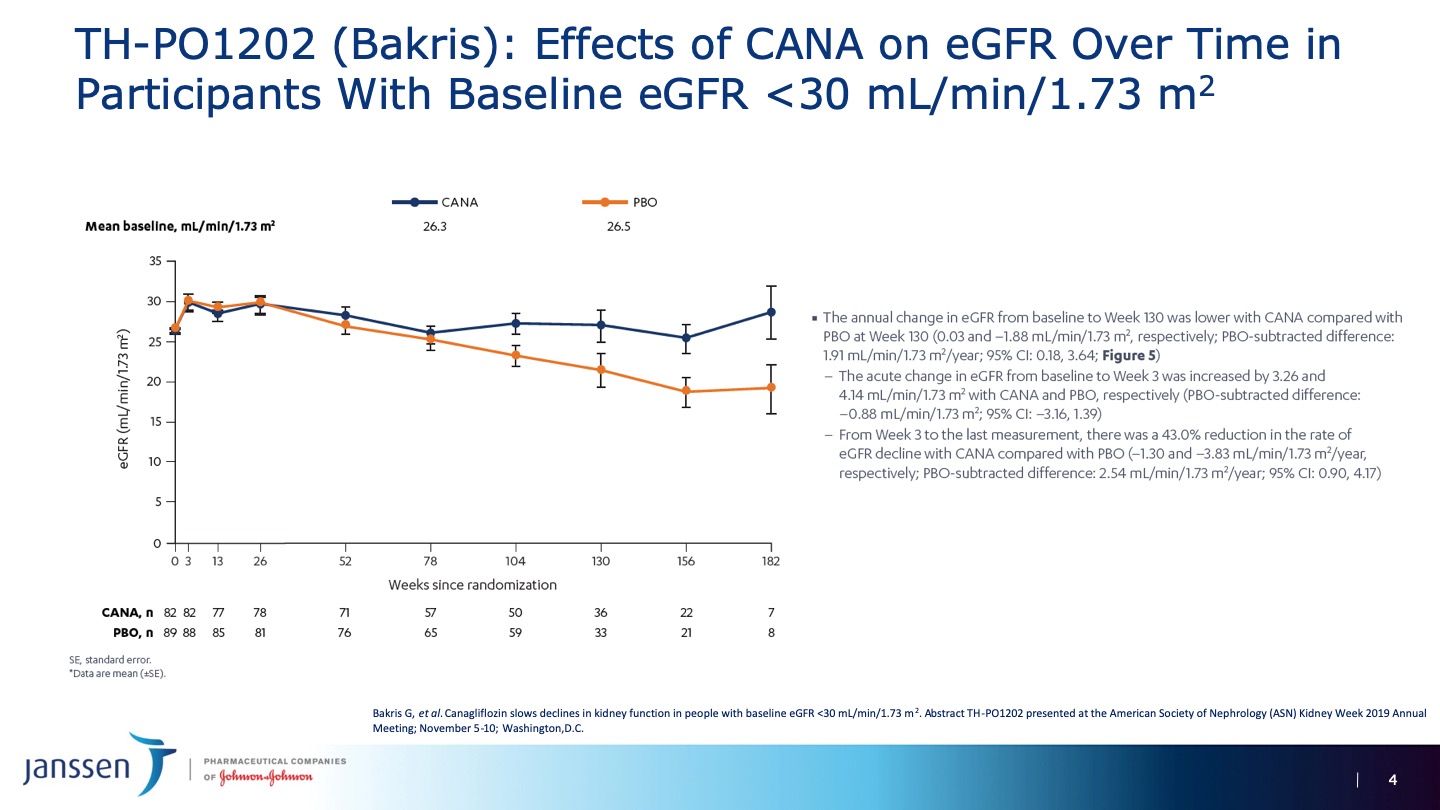
While we must excecise caution in applying the CREDENCE data to patients with characteristics unlike those in the study, this latest analysis - while small and of limited power - does suggest that patients with more significant GFR decline may also benefit from this extremely important medication. A randomized control trial focussing specifically on patients with severe CKD is likely warranted.
The key slides are included below for your reference:






