UKidney Nephrology News and Insights
Angiotensin Receptor Blockers and Cancer
 In the June 2010 online version of the Lancet Oncology journal, a provocative report of a recently completed meta-analysis suggests that angiotensin receptor blockers might confer a modest but statistically significant increased risk for cancer.
In the June 2010 online version of the Lancet Oncology journal, a provocative report of a recently completed meta-analysis suggests that angiotensin receptor blockers might confer a modest but statistically significant increased risk for cancer.
These results were a mixture of both prespecified and non prespecified cancer outcomes in clinical trials where different ARBs were used though telmisartan was the study drug in approximately 86% of patients. Patients randomly assigned to receive ARBs had a significantly increased risk of new cancer occurrence compared with patients in control groups (7·2% vs 6·0%, risk ratio RR 1·08, 95% CI 1·01—1·15; p=0·016). When analysis was limited to trials where cancer was a prespecified endpoint, the RR was 1·11 (95% CI 1·04—1·18, p=0·001).
This meta-analysis does suggest a modest but significant link between cancer use and ARBs. However, questions remain. Firstly, is this affect real? Secondly, can we generalize these results to other medications within the ARB class. An important limitation of this study is that much of the data were derived from the occurrence of cancer in patients from the ONTARGET Study in the treatment arm where both ramipril and telmisartan were used; there was no effect seen in the monotherapy arms.*
At this point, more study is required. It is tempting to draw conclusions from this meta-analysis, however, we must remember that research in this area is actually conflicting. For example, it has been suggested that ARBs can actually be protective against cancer, as seen here.
Therefore, further study is warranted before concluding that this very useful class of medications is harmful and whether this is a drug or class-specific effect.
*Below is a copy of the Forest plot for the meta-analysis. As you can see, the effect of telmisartan on cancer from ONTARGET (the largest study), was only present in the combination group, not when used alone:
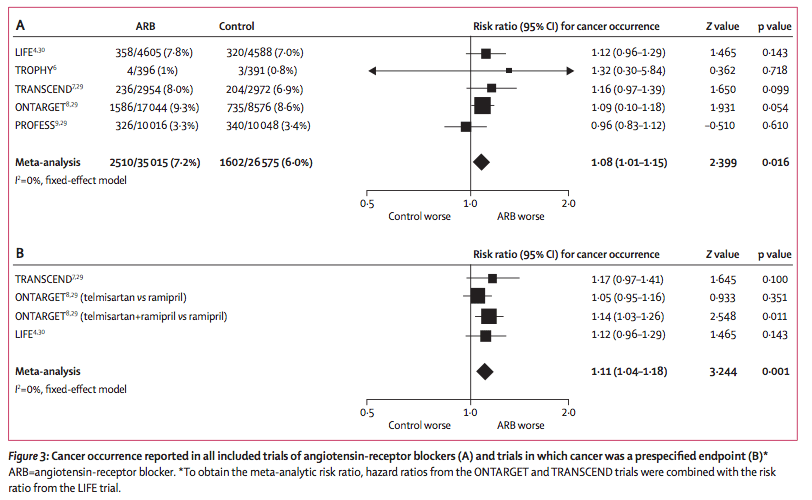
Source: The Lancet Oncology, Early Online Publication, 14 June 2010 doi:10.1016/S1470-2045(10)70106-6

