No Statistically Significant Difference in Cardiovascular and Renal Composite Endpoints Between Aranesp and Placebo
THOUSAND OAKS, Calif., Aug. 25 /PRNewswire-FirstCall/ -- Amgen (Nasdaq: AMGN) today announced that in a large, randomized, double-blind, placebo-controlled, Phase 3 study of patients with chronic kidney disease (CKD) (not requiring dialysis), anemia and type-2 diabetes (the Trial to Reduce CardiovascularEndpoints with Aranesp((R)) Therapy, or TREAT), treatment of anemia with Aranesp((R) )(darbepoetin alfa) to a hemoglobin target of 13 g/dL had no statistically significant effect on either of two primary endpoints compared with placebo treatment. The two primary endpoints were a composite of time to all-cause mortality or cardiovascular morbidity (including heart failure, heart attack, stroke, or hospitalization for myocardial ischemia) and a composite of time to all-cause mortality or chronic renal replacement therapy. Among the elements that formed these composite endpoints, an excess of stroke events (a labeled risk of Aranesp therapy) occurred in the Aranesp-treated patients compared to those receiving placebo.
These summary results will be followed by full efficacy and safety analyses, which will be shared with global regulatory authorities and presented at an upcoming medical meeting later this year.
"TREAT was designed to answer important questions about the effects of erythropoiesis-stimulating agents (ESAs) on cardiovascular and renal outcomes in patients with renal insufficiency and type-2 diabetes. It is by any measure the most comprehensive analysis that has ever been performed to examine the impact of anemia therapy in patients who do not yet require dialysis. The trial will provide nephrologists with important information as they endeavor to improve renal care," said Roger M. Perlmutter, M.D., Ph.D., executive vice president of Research and Development at Amgen. "In contrast to a recent, smaller study of ESAs in a similar patient population, TREAT did not show a statistically significant adverse effect on all-cause mortality or cardiovascular morbidity when patients were treated to a hemoglobin target of 13 g/dL. We continue to believe that ESAs have a favorable benefit:risk profile when used according to the approved label."
Currently, Aranesp is indicated for the treatment of anemia in patients with chronic renal failure (CRF), including patients on dialysis and patients not on dialysis. The approved label for Aranesp recommends individualizing dosing to achieve and maintain hemoglobin levels within the range of 10 to 12 g/dL. TREAT studied uses for Aranesp in which it is not approved.
TREAT Study Design
TREAT was an international, Phase 3, randomized, double-blind, placebo-controlled study of 4,038 chronic kidney disease (CKD) patients with type-2 diabetes and anemia. It is the largest study of ESA use in CKD patients to date. Patients enrolled in the study were randomized in a one-to-one ratio to receive either treatment with Aranesp to a target hemoglobin of 13 g/dL or placebo. Due to the increased risk of negative outcomes associated with low hemoglobin levels, patients in the control arm whose hemoglobin fell below 9 g/dL were given Aranesp until their hemoglobin level was 9 g/dL. Investigators were blinded to this intervention.
TREAT had two primary endpoints. The first evaluated time to all-cause mortality or cardiovascular morbidity including heart attack (myocardial infarction), congestive heart failure, hospitalization for angina (myocardial ischemia), or stroke (cerebrovascular accident). The second primary endpoint evaluated time to all-cause mortality or chronic dialysis. TREAT was not designed to determine the appropriate hemoglobin target in this patient population.
For patients randomized to the Aranesp group, the starting dose was 0.75 mcg/kg administered subcutaneously every two weeks; subsequent doses were titrated to achieve hemoglobin target of 13.0 g/dL. Once the target hemoglobin was reached, the frequency of administration was extended to once-monthly.
Chronic Kidney Disease: Impact and Prevalence
CKD affects more than 26 million Americans and millions more worldwide. The disease is characterized by progressive kidney damage and impaired kidney function and is most often caused by type-2 diabetes or high blood pressure. When CKD progresses to kidney failure, chronic dialysis or a kidney transplant are required to sustain life. Approximately 350,000 people in the United States are on dialysis today. Anemia is a common complication of CKD that may begin in the early stages of the disease and becomes more common and severe as kidney function declines. Studies have shown that anemia is associated with an increased risk of mortality and cardiovascular morbidity in CKD patients.
About Aranesp
Aranesp was approved by the U.S. Food and Drug Administration in 2001 for the treatment of anemia associated with CRF for patients on dialysis and patients not on dialysis. The European Commissiongranted marketing authorization for the same indication in 2001 and subsequently updated it for CRF patients with symptomatic anemia in 2008.
In 2002, the FDA approved the treatment of anemia caused by concomitantly administered chemotherapy in patients with nonmyeloid malignancies. The European Commission authorized the treatment of anemia caused by concomitantly administered chemotherapy in patients with non-haemological malignancies in 2002 and extended it to include non-myeloid malignancies in patients receiving chemotherapy in 2003.
Important Aranesp Safety Information
WARNINGS: INCREASED MORTALITY, SERIOUS CARDIOVASCULAR and THROMBOEMBOLIC EVENTS, and TUMOR PROGRESSION
Renal failure: Patients experienced greater risks for death and serious cardiovascular events when administered erythropoiesis-stimulating agents (ESAs) to target higher versus lower hemoglobin levels (13.5 vs. 11.3 g/dL; 14 vs. 10 g/dL) in two clinical studies. Individualize dosing to achieve and maintain hemoglobin levels within the range of 10 to 12 g/dL.
Cancer:
-- ESAs shortened overall survival and/or time-to-tumor progression in clinical studies in patients with breast, non-small cell lung, head and neck, lymphoid, and cervical cancers when dosed to target a hemoglobin of greater than or equal to 12 g/dL.
-- To minimize these risks, as well as the risk of serious cardio- and thrombovascular events, use the lowest dose needed to avoid red blood cell transfusions.
-- Use only for treatment of anemia due to concomitant myelosuppressive chemotherapy.
-- ESAs are not indicated for patients receiving myelosuppressive therapy when the anticipated outcome is cure. (This information is specific to the U.S. prescribing information)
-- Discontinue following the completion of a chemotherapy course.
Aranesp is contraindicated in patients with uncontrolled hypertension.
All patients, including patients with cancer or chronic kidney failure:
-- You may get serious heart problems such as heart attack, stroke, heart failure, and may die sooner if you are treated with Aranesp to a hemoglobin level above 12 g/dL.
-- You may get blood clots at any time while taking Aranesp. If you are receiving Aranesp and you are going to have surgery, talk to your healthcare provider about whether or not you need to take a blood thinner to lessen the chance of blood clots during or following surgery. Clots can form in blood vessels (veins), especially in your leg (deep venous thrombosis or DVT). Pieces of a blood clot may travel to the lungs and block the blood circulation in the lungs (pulmonary embolus).
About Amgen
Amgen discovers, develops, manufactures and delivers innovative human therapeutics. A biotechnology pioneer since 1980, Amgen was one of the first companies to realize the new science's promise by bringing safe and effective medicines from lab, to manufacturing plant, to patient. Amgen therapeutics have changed the practice of medicine, helping millions of people around the world in the fight against cancer, kidney disease, rheumatoid arthritis, and other serious illnesses. With a deep and broad pipeline of potential new medicines, Amgen remains committed to advancing science to dramatically improve people's lives. To learn more about our pioneering science and our vital medicines, visit www.amgen.com.
Forward-Looking Statements
This news release contains forward-looking statements that are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties and assumptions that could cause actual results to differ materially from those described. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements, including estimates of revenues, operating margins, capital expenditures, cash, other financial metrics, expected legal, arbitration, political, regulatory or clinical results or practices, customer and prescriber patterns or practices, reimbursement activities and outcomes and other such estimates and results. Forward-looking statements involve significant risks and uncertainties, including those discussed below and more fully described in theSecurities and Exchange Commission (SEC) reports filed by Amgen, including Amgen's most recent annual report on Form 10-K and most recent periodic reports on Form 10-Q and Form 8-K. Please refer toAmgen's most recent Forms 10-K, 10-Q and 8-K for additional information on the uncertainties and risk factors related to our business. Unless otherwise noted, Amgen is providing this information as of Aug. 25, 2009 and expressly disclaims any duty to update information contained in this news release.
No forward-looking statement can be guaranteed and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products after they are on the market. Our business may be impacted by government investigations, litigation and products liability claims. We depend on third parties for a significant portion of our manufacturing capacity for the supply of certain of our current and future products and limits on supply may constrain sales of certain of our current products and product candidate development.
In addition, sales of our products are affected by the reimbursement policies imposed by third-party payors, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment as well as U.S. legislation affecting pharmaceutical pricing and reimbursement. Government and others' regulations and reimbursement policies may affect the development, usage and pricing of our products. In addition, we compete with other companies with respect to some of our marketed products as well as for the discovery and development of new products. We believe that some of our newer products, product candidates or new indications for existing products, may face competition when and as they are approved and marketed. Our products may compete against products that have lower prices, established reimbursement, superior performance, are easier to administer, or that are otherwise competitive with our products. In addition, while we routinely obtain patents for our products and technology, the protection offered by our patents and patent applications may be challenged, invalidated or circumvented by our competitors and there can be no guarantee of our ability to obtain or maintain patent protection for our products or product candidates. We cannot guarantee that we will be able to produce commercially successful products or maintain the commercial success of our existing products. Our stock price may be affected by actual or perceived market opportunity, competitive position, and success or failure of our products or product candidates. Further, the discovery of significant problems with a product similar to one of our products that implicate an entire class of products could have a material adverse effect on sales of the affected products and on our business and results of operations.
The scientific information discussed in this news release related to our product candidates is preliminary and investigative. Such product candidates are not approved by the U.S. Food and Drug Administration(FDA), and no conclusions can or should be drawn regarding the safety or effectiveness of the product candidates. Only the FDA can determine whether the product candidates are safe and effective for the use(s) being investigated. Further, the scientific information discussed in this news release relating to new indications for our products is preliminary and investigative and is not part of the labeling approved by theU.S. Food and Drug Administration (FDA) for the products. The products are not approved for the investigational use(s) discussed in this news release, and no conclusions can or should be drawn regarding the safety or effectiveness of the products for these uses. Only the FDA can determine whether the products are safe and effective for these uses. Healthcare professionals should refer to and rely upon the FDA-approved labeling for the products, and not the information discussed in this news release.
SOURCE Amgen
 Here is a summary of the key findings:
Here is a summary of the key findings: I have never been a fan of vitamins. They fall in to a category of interventions with presumed safety and benefit. An important study in the
I have never been a fan of vitamins. They fall in to a category of interventions with presumed safety and benefit. An important study in the 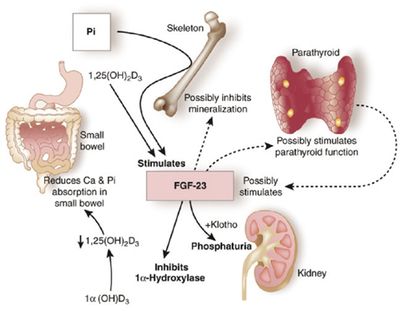 atemia has been been linked to poor patient outcomes, including a link to higher mortality. This relationship has been inferred by several retrospective and observational studies. In fact, the relationship between hyperphosphatemia and death is one of the most consistently espoused theories in all of nephrology. There is just problem however; there has never been a randomized trial to confirm this association.
atemia has been been linked to poor patient outcomes, including a link to higher mortality. This relationship has been inferred by several retrospective and observational studies. In fact, the relationship between hyperphosphatemia and death is one of the most consistently espoused theories in all of nephrology. There is just problem however; there has never been a randomized trial to confirm this association.