 Imagining the Dream RCT in nephrology is an important exercise, but not for the reasons you might think. For many, there might be the temptation to devise trials that will pave the way for future enhancements to patient care or to some other exciting, forward thinking outcome. For me however, the Dream RCT in nephrology is about shattering or affirming nephrology dogma - and in nephrology we seem to have more dogma influencing core tenets of our specialty than in other medical disciplines. There is a popular saying among those in the clinical epidemiology world - that you don’t need to conduct an RCT to prove that parachutes are required when jumping out of a plane. Unfortunately in nephrology, thought leaders have bestowed parachute status on too many interventions. I can think of no better example for this analogy than treating hyperphosphatemia in CKD/ESRD. And it is this topic that would underlie my Dream RCT.
Imagining the Dream RCT in nephrology is an important exercise, but not for the reasons you might think. For many, there might be the temptation to devise trials that will pave the way for future enhancements to patient care or to some other exciting, forward thinking outcome. For me however, the Dream RCT in nephrology is about shattering or affirming nephrology dogma - and in nephrology we seem to have more dogma influencing core tenets of our specialty than in other medical disciplines. There is a popular saying among those in the clinical epidemiology world - that you don’t need to conduct an RCT to prove that parachutes are required when jumping out of a plane. Unfortunately in nephrology, thought leaders have bestowed parachute status on too many interventions. I can think of no better example for this analogy than treating hyperphosphatemia in CKD/ESRD. And it is this topic that would underlie my Dream RCT.
UKidney Nephrology News and Insights
Top-line results for the SAVOR trial were announced this week in which Saxagliptin (Onglyza, Bristol-Myers Squibb/AstraZeneca) failed to demonstrate superiority over placebo in reducing a composite end point of cardiovascular death, nonfatal MI, or nonfatal ischemic stroke when added to usual care in patients with type 2 diabetes with either a history of established CVD or multiple CVD risk factors. While the trial did demonstrate non-inferiority from a cardiovascular safety perspective, it failed to show any efficacy advantage. As reported originally on theheart.org:
Princeton, NJ and Wilmington, DE - The SAVOR-TIMI 53 trial has failed to demonstrate the superiority ofsaxagliptin (Onglyza, Bristol-Myers Squibb/AstraZeneca) over placebo in reducing a composite end point of cardiovascular death, nonfatal MI, or nonfatal ischemic stroke when added to usual care in patients with type 2 diabetes with either a history of established CVD or multiple CVD risk factors [1].
The trial sponsors, Bristol-Myers Squibb and AstraZeneca, announced the top-line results from the trial early this morning. The full findings will be presented September 2, 2013 at the European Society of Cardiology(ESC) 2013 Congress. The trial did meet its "primary safety objective of noninferiority" vs placebo, a joint statement from the companies reads. SAVOR-TIMI-53 is part of an ESC hot-line session dedicated to risk factors and diabetes. The full schedule of ESC 2013 hot lines was published on the ESC website earlier this week.
Saxagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor approved in the US, Canada, Europe, and elsewhere as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes. Other, nonrandomized analyses had raised hopes that this class of drugs might have a protective effect on the vasculature of diabetes patients.
Saxagliptin was the first new diabetes drug to receive FDA approval after the issuance of new agency guidelines in July 2009, requiring companies to perform CV-outcomes studies with new diabetes drugs. The drug's clinical development program had been completed before the guidance, but because of the new rule, the company launched SAVOR-TIMI 53. The four-year-long trial had a target enrollment of 16 500 patients, and principal investigators for the trial are Dr Itamar Raz (Hadassah Medical Organization, Jerusalem, Israel) and Dr Deepak Bhatt (Brigham and Women's Hospital, Boston, MA).
Some very disappointing news released today, as seen on Reuters:
Oct 18 (Reuters) - Abbott Laboratories Inc said its partner Reata Pharmaceuticals was discontinuing a late-stage trial of their potential blockbuster treatment for chronic kidney disease and diabetes based on safety concerns raised by an independent safety committee. The news for bardoxolone represents a major setback for Abbott just months before the planned Jan. 1 spinoff of its branded prescription drugs into a separate publicly traded company called AbbVie. Without the high-profile drug, Wall Street concerns about AbbVie's dependence on Abbott's $8 billion-a-year rheumatoid arthritis drug Humira could intensify. An independent data monitoring committee found excess serious adverse events and mortality in patients taking the oral anti-inflammatory drug, Abbott said in a regulatory filing.
Regulators were notified of the decision, and study participants were being informed, the company said.
At the end of 2011, Novartis suspended the ALTITUDE Study, effectively ending the possibility that the direct renin inhibitor (DRI) Aliskiren will play a significant role in the management of patients with CKD already on an ACE or ARB. This development was disappointing but actually represents only part of a growing body of evidence that now casts major doubt on the use of microalbuminuria (MAU) as a treatment surrogate in patients with cardiovascular disease.
The use of MAU as a predictor of cardiovascular risk is sound and supported by a sizable evidence base. There is little doubt that patients with risks for cardiovascular disease who also have MAU are at far greater risk for adverse outcomes including death. In numerous studies, including the Heart Outcomes Prevention Evaluation (HOPE) Trial [7], MAU was the single most potent risk factor for adverse outcomes, with greater predictive power than diabetes, male gender, smoking and hypertension. The fascinating part of this observation is that even seemingly modest elevation in MAU was highly predictive of adverse events. It was very tempting then to anticipate a concomitant reduction in risk among patients whose MAU was targeted for therapeutic reduction with any of: (1) high dose ACE or ARB, (2) combination ACE and ARB, and most recently, (3) combination ACE or ARB with DRI. Unfortunately, recent prospective studies have essentially decimated this hypothesis and in all, proteinuria improved whereas patients did not or actually fared worse.
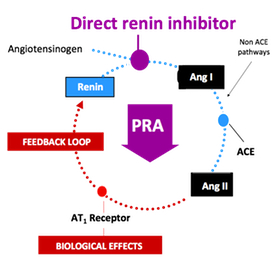 In a stunning development, Novartis said Tuesday that it will terminate the late-stage ALTITUDE study investigating Rasilez (aliskiren) in patients with type 2 diabetes and renal impairment on the recommendation of an independent data monitoring committee. The company indicated that the committee concluded that "patients were unlikely to benefit" from the addition of Rasilez to standard anti-hypertensives and also identified higher adverse events in this group (source: FirstWord).
In a stunning development, Novartis said Tuesday that it will terminate the late-stage ALTITUDE study investigating Rasilez (aliskiren) in patients with type 2 diabetes and renal impairment on the recommendation of an independent data monitoring committee. The company indicated that the committee concluded that "patients were unlikely to benefit" from the addition of Rasilez to standard anti-hypertensives and also identified higher adverse events in this group (source: FirstWord).
On a personal and professional note, these results come as a great disappointment to me. Not only does it suggest no further protection for our patients prescribed this strategy in an effort to reduce the burden of cardiorenal disease, but I was a great believer in the hypothesis and taught about it extensively throughout my career.
In this week's New England Journal of Medicine, Pergola et al report the results of a phase 2 randomized trial of an antioxidant inflammation modulator, bardoxolone, in patients with type 2 diabetes and chronic kidney disease (CKD). Over a period of 52 weeks, 227 adults with CKD (defined as an estimated glomerular filtration rate [GFR] of 20 to 45 ml per minute per 1.73 m2 of body-surface area) were randomized in a 1:1:1:1 ratio to receive placebo or bardoxolone methyl at a target dose of 25, 75, or 150 mg once daily. Primary outcome was a change from baseline in the estimated GFR with bardoxolone, as compared with placebo, at 24 weeks; a secondary outcome was the change at 52 weeks. The results were quite surprising.
This is a great post on chloroquine in lupus. It appears as below on Skin and Allergy News. A great and promising read:
SNOWMASS, Colo. – The past 12 months have brought a slew of studies making a persuasive case for hydroxychloroquine as a far more important drug in lupus than previously thought. Indeed, the drug could now even be considered essential.
"In 2011, all lupus patients should receive hydroxychloroquine," Dr. David Wofsy flatly declared at a symposium sponsored by the American College of Rheumatology.
"The indication for hydroxychloroquine in lupus is lupus," added Dr. Wofsy, professor of medicine and microbiology/immunology at the University of California, San Francisco.
There is now solid evidence that hydroxychloroquine (Plaquenil) prevents lupus flares, treats the skin manifestations of the disease, protects against thromboembolic events, prevents cardiac neonatal lupus, and prolongs life.
"It will be a very long time before we've proven that any biologic therapy can do all those things," Dr. Wofsy, who is also chief of rheumatology at the San Francisco Veterans Affairs Medical Center.
The Planet 1 and 2 studies are not widely publicized. However, they do provide some eye-opening information.
According to these studies, in patients with diabetes and intact renal function, there was a significant decline in renal function and failure to reduce urinary protein in patients randomized to rosuvastatin but not atorvastatin. Furthermore, there were more renal events in the rosuvastatin group (doubling of serum creatinine and episodes of acute renal failure).
These results are very surprising and difficult to rationalize at face value. Nevertheless, the design of the trials appears sound and the number of patients adequate.
Dr. Marecllo Tonelli from the University of Alberta walks us through this data in his outstanding presentation seen here on UKidney
The SHARP study was reported at the American Society of Nephrology Meeting in Denver on November 20th, 2010. The results are positive. This is excellent news indeed when previous lipid trials in patients with renal failure were a disappointment.
 Here is a summary of the key findings:
Here is a summary of the key findings:
- The patients allocated to take ezetimibe plus simvastatin had one-sixth fewer heart attacks, strokes or operations to unblock arteries ("major atherosclerotic events"), with similar reductions observed in all types of patient studied.
- During this long trial, the proportion of patients who stopped taking their allocated treatment was about one third, but this was not generally due to side-effects and was the same for both real and dummy treatments. If taken without interruption, however, ezetimibe plus simvastatin could have even larger effects than were seen in SHARP, potentially reducing risk by about one quarter.
- Adding 10mg daily of ezetimibe to 20mg daily of simvastatin produced a large reduction in LDL cholesterol safely. This combination treatment may be particularly good for kidney patients, as it avoids the possibility of side-effects with high statin doses.
- There was no support for previous concerns with ezetimibe about possible adverse effects on cancer, and no evidence of an increased risk of muscle or liver problems.
(Source: http://ukid.cc/atgiPN)
As with any study, a complete critical appraisal should be done on the published article once available. One key question is whether the observed benefit was the result of ezetimibe or simply the result of lower LDL in the treatment group, regardles how obtained (i.e. with higher statin dosing).

Any news on promising treatments for Lupus is always welcome. Indeed, it has been years since any new therapies have provided much new hope for a disease which has very significant renal implications.
Benlysta is an investigational human monoclonal antibody drug and the first in a new class of drugs called BLyS-specific inhibitors. These drugs prevent b-cell proliferation and development in to mature plasma cells with a resuling drop in antibody production. This mechanism of action is very well-suited to Lupus whose pathophysiology is widely thought to involve autoantibody formation.
Results of phase-3 clinical trials have been previously reported showing positive results, a first in many years for the management of lupus. As a result, the drug has been granted a priority review designation by the FDA (Food and Drug Administration, USA), an indication that this drug has an important role in managing lupus as other medications have left much to be desired.
What is not clear however, is the role that this medication will have in the management of lupus nephritis as it seems that these patients were excluded from the initial trials.
{youtube}DeauD2aI_fM{/youtube}
 I have never been a fan of vitamins. They fall in to a category of interventions with presumed safety and benefit. An important study in the Journal of the American Medical Association shows the opposite; that vitamins can cause harm in patients with chronic kidney disease.
I have never been a fan of vitamins. They fall in to a category of interventions with presumed safety and benefit. An important study in the Journal of the American Medical Association shows the opposite; that vitamins can cause harm in patients with chronic kidney disease.
The following appears on the BC Renal Agency Website:
In April, the Journal of the American Medical Association (JAMA) published a study that looked at whether high doses of B vitamins (folic acid, B12, B6) helped people with kidney disease due to diabetes. The study found that high doses of these vitamins were actually harmful. Study participants who took the vitamins had an increased risk of heart attack and stroke. They also had reduced kidney function.
 Many studies have questioned the effectiveness of hydrochlorothiazide (HCTZ) versus chlorthalidone as a diuretic. In fact, most large scale trials that have used HCTZ have been disappointing (e.g. ACCOMPLISH) while those using chlorthalidone have been largely positive (e.g. ALLHAT). While this might seem like an over-simplification, many hypertension experts agree with it.
Many studies have questioned the effectiveness of hydrochlorothiazide (HCTZ) versus chlorthalidone as a diuretic. In fact, most large scale trials that have used HCTZ have been disappointing (e.g. ACCOMPLISH) while those using chlorthalidone have been largely positive (e.g. ALLHAT). While this might seem like an over-simplification, many hypertension experts agree with it.
In the latest twist to this story, Takeda Pharmaceuticals have created a fixed dose combination with it's new ARB azilsartan with chlorthalidone - in stark contrast to all other ARB and ACE inhibitor counterparts. As it it turns out, they may be on to something ( continued ... )
ScienceDaily (May 6, 2010) — Two-year results from phase III clinical trials show the experimental immunosuppressive drug belatacept can better preserve kidney function in kidney transplant recipients while preventing graft rejection when compared with the standard immunosuppressive drug cyclosporine.
 Over the past 2 years, considerable excitement has been building over the results of the ACCOMPLISH study. This trial suggested that the combination of benazapril plus amlodipine is superior to benazapril plus hydrochlorothiazide for the prevention of a composite cardiovascular outcome. While there are methodological concerns regarding this trial that make me question its generalizability, it is thought-provoking to consider that one medication combination is superior to another even if blood-pressure between the 2 groups is negligible.
Over the past 2 years, considerable excitement has been building over the results of the ACCOMPLISH study. This trial suggested that the combination of benazapril plus amlodipine is superior to benazapril plus hydrochlorothiazide for the prevention of a composite cardiovascular outcome. While there are methodological concerns regarding this trial that make me question its generalizability, it is thought-provoking to consider that one medication combination is superior to another even if blood-pressure between the 2 groups is negligible.
In the latest issue of Lancet, a follow-up paper suggests that benzapril-amlodipine prevented renal outcomes more-so than in the benazapril-hydrochlorothiazide arm. However, as the excellent accompanying editorial points out, all is not as it appears. (continued...)
Hyperphosph atemia has been been linked to poor patient outcomes, including a link to higher mortality. This relationship has been inferred by several retrospective and observational studies. In fact, the relationship between hyperphosphatemia and death is one of the most consistently espoused theories in all of nephrology. There is just problem however; there has never been a randomized trial to confirm this association.
atemia has been been linked to poor patient outcomes, including a link to higher mortality. This relationship has been inferred by several retrospective and observational studies. In fact, the relationship between hyperphosphatemia and death is one of the most consistently espoused theories in all of nephrology. There is just problem however; there has never been a randomized trial to confirm this association.
In the latest issue of Nephrology Dialysis and Transplantation, Smith et al cast doubt on this long-held belief. In their retrospective CKD-inception cohort study, there was no association between hyperphosphatemia and death, though there was less risk of renal replacement therapy in patients with better phosphorus control.
This finding is by no means conclusive. I continue to aggressively treat hyperphosphatemia. However, it does lend further support for a large-scale randomized trial to study this seemingly unimpeachable belief.
 The TREAT Trial was presented at the ASN in San Diego. This much publicized trial examined the role of Aranesp in the management of diabetic patients with CKD. One group was randomized to receive Aranesp with a hemoglobin target of 130 g/l while the control group was given placebo and treated with Aranesp only if their hemoglobin fell below 90 g/l.
The TREAT Trial was presented at the ASN in San Diego. This much publicized trial examined the role of Aranesp in the management of diabetic patients with CKD. One group was randomized to receive Aranesp with a hemoglobin target of 130 g/l while the control group was given placebo and treated with Aranesp only if their hemoglobin fell below 90 g/l.
There was no advantage to the group given Aranesp and a statistically significant increase in strokes observed in the the treatment group. Below is a link to the article from NEJM and the accompanying editorial written by renowned nephrologist, Dr. Phil Marsden of the University of Toronto.
It is worth noting, that the treatment group was targeted to a higher hemoglobin than we now conventionally use. Furthermore, the dose of Aranesp was more than double the typical dosages used by the majority of my predialysis patients. As has been observed in other studies where high hemoglobin seems harmful, the dose of Aranesp required might explain the observation of harm. Perhaps those patients who respond to lower dosages and those whose hemoglobins remain with target would be perfectly safe to continue.
More study is needed to clarify this and perhaps might be forthcoming from further analysis of the TREAT Study.
Click for:
A Trial of Darbepoetin Alfa in Type 2 Diabetes and Chronic Kidney Disease
Treatment of Anemia in Chronic Kidney Disease — Strategies Based on Evidence
No Statistically Significant Difference in Cardiovascular and Renal Composite Endpoints Between Aranesp and Placebo
THOUSAND OAKS, Calif., Aug. 25 /PRNewswire-FirstCall/ -- Amgen (Nasdaq: AMGN) today announced that in a large, randomized, double-blind, placebo-controlled, Phase 3 study of patients with chronic kidney disease (CKD) (not requiring dialysis), anemia and type-2 diabetes (the Trial to Reduce CardiovascularEndpoints with Aranesp((R)) Therapy, or TREAT), treatment of anemia with Aranesp((R) )(darbepoetin alfa) to a hemoglobin target of 13 g/dL had no statistically significant effect on either of two primary endpoints compared with placebo treatment. The two primary endpoints were a composite of time to all-cause mortality or cardiovascular morbidity (including heart failure, heart attack, stroke, or hospitalization for myocardial ischemia) and a composite of time to all-cause mortality or chronic renal replacement therapy. Among the elements that formed these composite endpoints, an excess of stroke events (a labeled risk of Aranesp therapy) occurred in the Aranesp-treated patients compared to those receiving placebo.
These summary results will be followed by full efficacy and safety analyses, which will be shared with global regulatory authorities and presented at an upcoming medical meeting later this year.
"TREAT was designed to answer important questions about the effects of erythropoiesis-stimulating agents (ESAs) on cardiovascular and renal outcomes in patients with renal insufficiency and type-2 diabetes. It is by any measure the most comprehensive analysis that has ever been performed to examine the impact of anemia therapy in patients who do not yet require dialysis. The trial will provide nephrologists with important information as they endeavor to improve renal care," said Roger M. Perlmutter, M.D., Ph.D., executive vice president of Research and Development at Amgen. "In contrast to a recent, smaller study of ESAs in a similar patient population, TREAT did not show a statistically significant adverse effect on all-cause mortality or cardiovascular morbidity when patients were treated to a hemoglobin target of 13 g/dL. We continue to believe that ESAs have a favorable benefit:risk profile when used according to the approved label."
Currently, Aranesp is indicated for the treatment of anemia in patients with chronic renal failure (CRF), including patients on dialysis and patients not on dialysis. The approved label for Aranesp recommends individualizing dosing to achieve and maintain hemoglobin levels within the range of 10 to 12 g/dL. TREAT studied uses for Aranesp in which it is not approved.
TREAT Study Design
TREAT was an international, Phase 3, randomized, double-blind, placebo-controlled study of 4,038 chronic kidney disease (CKD) patients with type-2 diabetes and anemia. It is the largest study of ESA use in CKD patients to date. Patients enrolled in the study were randomized in a one-to-one ratio to receive either treatment with Aranesp to a target hemoglobin of 13 g/dL or placebo. Due to the increased risk of negative outcomes associated with low hemoglobin levels, patients in the control arm whose hemoglobin fell below 9 g/dL were given Aranesp until their hemoglobin level was 9 g/dL. Investigators were blinded to this intervention.
TREAT had two primary endpoints. The first evaluated time to all-cause mortality or cardiovascular morbidity including heart attack (myocardial infarction), congestive heart failure, hospitalization for angina (myocardial ischemia), or stroke (cerebrovascular accident). The second primary endpoint evaluated time to all-cause mortality or chronic dialysis. TREAT was not designed to determine the appropriate hemoglobin target in this patient population.
For patients randomized to the Aranesp group, the starting dose was 0.75 mcg/kg administered subcutaneously every two weeks; subsequent doses were titrated to achieve hemoglobin target of 13.0 g/dL. Once the target hemoglobin was reached, the frequency of administration was extended to once-monthly.
Chronic Kidney Disease: Impact and Prevalence
CKD affects more than 26 million Americans and millions more worldwide. The disease is characterized by progressive kidney damage and impaired kidney function and is most often caused by type-2 diabetes or high blood pressure. When CKD progresses to kidney failure, chronic dialysis or a kidney transplant are required to sustain life. Approximately 350,000 people in the United States are on dialysis today. Anemia is a common complication of CKD that may begin in the early stages of the disease and becomes more common and severe as kidney function declines. Studies have shown that anemia is associated with an increased risk of mortality and cardiovascular morbidity in CKD patients.
About Aranesp
Aranesp was approved by the U.S. Food and Drug Administration in 2001 for the treatment of anemia associated with CRF for patients on dialysis and patients not on dialysis. The European Commissiongranted marketing authorization for the same indication in 2001 and subsequently updated it for CRF patients with symptomatic anemia in 2008.
In 2002, the FDA approved the treatment of anemia caused by concomitantly administered chemotherapy in patients with nonmyeloid malignancies. The European Commission authorized the treatment of anemia caused by concomitantly administered chemotherapy in patients with non-haemological malignancies in 2002 and extended it to include non-myeloid malignancies in patients receiving chemotherapy in 2003.
Important Aranesp Safety Information
WARNINGS: INCREASED MORTALITY, SERIOUS CARDIOVASCULAR and THROMBOEMBOLIC EVENTS, and TUMOR PROGRESSION
Renal failure: Patients experienced greater risks for death and serious cardiovascular events when administered erythropoiesis-stimulating agents (ESAs) to target higher versus lower hemoglobin levels (13.5 vs. 11.3 g/dL; 14 vs. 10 g/dL) in two clinical studies. Individualize dosing to achieve and maintain hemoglobin levels within the range of 10 to 12 g/dL.
Cancer:
-- ESAs shortened overall survival and/or time-to-tumor progression in clinical studies in patients with breast, non-small cell lung, head and neck, lymphoid, and cervical cancers when dosed to target a hemoglobin of greater than or equal to 12 g/dL.
-- To minimize these risks, as well as the risk of serious cardio- and thrombovascular events, use the lowest dose needed to avoid red blood cell transfusions.
-- Use only for treatment of anemia due to concomitant myelosuppressive chemotherapy.
-- ESAs are not indicated for patients receiving myelosuppressive therapy when the anticipated outcome is cure. (This information is specific to the U.S. prescribing information)
-- Discontinue following the completion of a chemotherapy course.
Aranesp is contraindicated in patients with uncontrolled hypertension.
All patients, including patients with cancer or chronic kidney failure:
-- You may get serious heart problems such as heart attack, stroke, heart failure, and may die sooner if you are treated with Aranesp to a hemoglobin level above 12 g/dL.
-- You may get blood clots at any time while taking Aranesp. If you are receiving Aranesp and you are going to have surgery, talk to your healthcare provider about whether or not you need to take a blood thinner to lessen the chance of blood clots during or following surgery. Clots can form in blood vessels (veins), especially in your leg (deep venous thrombosis or DVT). Pieces of a blood clot may travel to the lungs and block the blood circulation in the lungs (pulmonary embolus).
About Amgen
Amgen discovers, develops, manufactures and delivers innovative human therapeutics. A biotechnology pioneer since 1980, Amgen was one of the first companies to realize the new science's promise by bringing safe and effective medicines from lab, to manufacturing plant, to patient. Amgen therapeutics have changed the practice of medicine, helping millions of people around the world in the fight against cancer, kidney disease, rheumatoid arthritis, and other serious illnesses. With a deep and broad pipeline of potential new medicines, Amgen remains committed to advancing science to dramatically improve people's lives. To learn more about our pioneering science and our vital medicines, visit www.amgen.com.
Forward-Looking Statements
This news release contains forward-looking statements that are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties and assumptions that could cause actual results to differ materially from those described. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements, including estimates of revenues, operating margins, capital expenditures, cash, other financial metrics, expected legal, arbitration, political, regulatory or clinical results or practices, customer and prescriber patterns or practices, reimbursement activities and outcomes and other such estimates and results. Forward-looking statements involve significant risks and uncertainties, including those discussed below and more fully described in theSecurities and Exchange Commission (SEC) reports filed by Amgen, including Amgen's most recent annual report on Form 10-K and most recent periodic reports on Form 10-Q and Form 8-K. Please refer toAmgen's most recent Forms 10-K, 10-Q and 8-K for additional information on the uncertainties and risk factors related to our business. Unless otherwise noted, Amgen is providing this information as of Aug. 25, 2009 and expressly disclaims any duty to update information contained in this news release.
No forward-looking statement can be guaranteed and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products after they are on the market. Our business may be impacted by government investigations, litigation and products liability claims. We depend on third parties for a significant portion of our manufacturing capacity for the supply of certain of our current and future products and limits on supply may constrain sales of certain of our current products and product candidate development.
In addition, sales of our products are affected by the reimbursement policies imposed by third-party payors, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment as well as U.S. legislation affecting pharmaceutical pricing and reimbursement. Government and others' regulations and reimbursement policies may affect the development, usage and pricing of our products. In addition, we compete with other companies with respect to some of our marketed products as well as for the discovery and development of new products. We believe that some of our newer products, product candidates or new indications for existing products, may face competition when and as they are approved and marketed. Our products may compete against products that have lower prices, established reimbursement, superior performance, are easier to administer, or that are otherwise competitive with our products. In addition, while we routinely obtain patents for our products and technology, the protection offered by our patents and patent applications may be challenged, invalidated or circumvented by our competitors and there can be no guarantee of our ability to obtain or maintain patent protection for our products or product candidates. We cannot guarantee that we will be able to produce commercially successful products or maintain the commercial success of our existing products. Our stock price may be affected by actual or perceived market opportunity, competitive position, and success or failure of our products or product candidates. Further, the discovery of significant problems with a product similar to one of our products that implicate an entire class of products could have a material adverse effect on sales of the affected products and on our business and results of operations.
The scientific information discussed in this news release related to our product candidates is preliminary and investigative. Such product candidates are not approved by the U.S. Food and Drug Administration(FDA), and no conclusions can or should be drawn regarding the safety or effectiveness of the product candidates. Only the FDA can determine whether the product candidates are safe and effective for the use(s) being investigated. Further, the scientific information discussed in this news release relating to new indications for our products is preliminary and investigative and is not part of the labeling approved by theU.S. Food and Drug Administration (FDA) for the products. The products are not approved for the investigational use(s) discussed in this news release, and no conclusions can or should be drawn regarding the safety or effectiveness of the products for these uses. Only the FDA can determine whether the products are safe and effective for these uses. Healthcare professionals should refer to and rely upon the FDA-approved labeling for the products, and not the information discussed in this news release.
SOURCE Amgen
In the latest edition of the Journal American Association of Nephrology, investigators report that sodium bicarbonate administration delays the progression of kidney disease to end-stage renal failure. 134 adult patients with CKD (creatinine clearance [CrCl] 15 to 30 ml/min per 1.73 m2) and serum bicarbonate 16 to 20 mmol/L to either supplementation with oral sodium bicarbonate or standard care for 2 yr. With this simple intervention, patients receiving sodium bicarbonate were significantly less likely to experience rapid progression (9 versus 45%; relative risk 0.15; 95% confidence interval 0.06 to 0.40; P < 0.0001). Compared with the control group, decline in CrCl was slower with bicarbonate supplementation (5.93 versus 1.88 ml/min 1.73 m2; P < 0.0001). Nutritional parameters were improved as well. This very simple intervention, practiced variably by nephrologists, shows great promise in the management of patients with chronic kidney disease.
Published: May 12, 2009
Reviewed by Zalman S. Agus, MD; Emeritus Professor
University of Pennsylvania School of Medicine.
SAN FRANCISCO, May 12 -- Ambulatory blood pressure monitoring didn't explain the cardiovascular advantage of calcium channel blockade found in the ACCOMPLISH trial, researchers said.
The primary findings of that trial revealed a 20% reduction in cardiovascular mortality and morbidity with the calcium channel blocker amlodipine (Norvasc) versus the diuretic hydrochlorothiazide (Microzide) as the initial antihypertensive in combination with the ACE inhibitor benazepril (Lotensin).
But in a secondary analysis of ACCOMPLISH results, 24-hour blood pressure monitoring revealed no difference in blood pressure control between the regimens, Kenneth Jamerson, M.D., of the University of Michigan in Ann Arbor, and colleagues found.
These results affirm that the calcium channel blocker combination has some "pleiotropic" benefits beyond blood pressure lowering alone, Dr. Jamerson reported at the American Society of Hypertension meeting.
Action Points
* Note that guidelines from the National Heart, Lung, and Blood Institute (JNC 7) recommend thiazide-type diuretics as initial therapy for most hypertensive patients, whether alone or in combination with an agent from another class.
* Note that this study was published as an abstract and presented orally at a conference. These data and conclusions should be considered to be preliminary until published in a peer-reviewed journal.
"It really does matter what agent you use," he said.
After the primary report of the data, concerns had arisen that lower blood pressure in the calcium channel blocker group biased the results, commented co-author George Bakris, M.D., of the University of Chicago, who moderated a press conference at which the findings were presented. (See ACC: Calcium Channel Blocker Beats Diuretic for Initial BP Combo Therapy)
Also, the trial used hydrochlorothiazide rather than the longer-acting diuretic chlorthalidone, which could have meant less blood pressure control over the full 24 hours compared with the other combination regimen.
But the ambulatory blood pressure results lay these questions to rest, Dr. Jamerson said.
"This type of data has the potential to change the paradigm to treat blood pressure from mostly being diuretic-based combination therapy to being amlodipine with benazipril type regimens," he said.
In the analysis of 573 patients in ACCOMPLISH, the in-clinic systolic blood pressure after two years of treatment averaged 0.6 mm Hg lower with amlodipine plus benazipril compared with hydrochlorothiazide plus benazipril (129.7 versus 130.3 mm Hg, P=0.621).
But the 24-hour blood pressure average actually favored the diuretic-ACE combination (122.3 versus 123.9 mm Hg, P=0.128), as did daytime and nighttime averages (P=0.097 and P=0.332).
For diastolic pressure, the diuretic combination also had a small, 0.3-mm Hg advantage over 24 hours (P=0.7).
None of these were significant differences, and both groups attained greater than 80% blood pressure control rates (81.3% with the calcium channel blocker and 84.9% with the diuretic combination, P=0.243).
Dr. Bakris said that a calcium channel blocker may have "pleiotropic" benefits for endothelial function and the atherosclerotic process that may have lowered cardiovascular risk despite similar blood pressure.
However, some at the late-breaking clinical trials session where the research was presented were skeptical.
Marvin Moser, M.D., of Yale University, who moderated the session, cautioned that the conclusions of the trial may have been overstated.
"The weight of data suggests it's the blood pressure level and not the specific drug," he said.
Guidelines from the National Heart, Lung, and Blood Institute (JNC 7) recommend thiazide-type diuretics as initial therapy for most hypertensive patients, whether alone or in combination with an agent from another class.
"Diuretics have held up as well as anything else," Dr. Moser said. "Before we abandon them we need further confirmation."
Another study presented at the same session, on which Dr. Bakris was also a co-author, suggested there was no difference between agents for left ventricular hypertrophy regression. (See ASH: Lower Blood Pressure Trumps Regimen in LV Remodeling)
Dr. Bakris noted that this surrogate endpoint may be important, but doesn't capture broader cardiovascular effects or the more important mortality endpoint.
