 I have never been a fan of vitamins. They fall in to a category of interventions with presumed safety and benefit. An important study in the Journal of the American Medical Association shows the opposite; that vitamins can cause harm in patients with chronic kidney disease.
I have never been a fan of vitamins. They fall in to a category of interventions with presumed safety and benefit. An important study in the Journal of the American Medical Association shows the opposite; that vitamins can cause harm in patients with chronic kidney disease.
The following appears on the BC Renal Agency Website:
In April, the Journal of the American Medical Association (JAMA) published a study that looked at whether high doses of B vitamins (folic acid, B12, B6) helped people with kidney disease due to diabetes. The study found that high doses of these vitamins were actually harmful. Study participants who took the vitamins had an increased risk of heart attack and stroke. They also had reduced kidney function.
 Imagining the Dream RCT in nephrology is an important exercise, but not for the reasons you might think. For many, there might be the temptation to devise trials that will pave the way for future enhancements to patient care or to some other exciting, forward thinking outcome. For me however, the Dream RCT in nephrology is about shattering or affirming nephrology dogma - and in nephrology we seem to have more dogma influencing core tenets of our specialty than in other medical disciplines. There is a popular saying among those in the clinical epidemiology world - that you don’t need to conduct an RCT to prove that parachutes are required when jumping out of a plane. Unfortunately in nephrology, thought leaders have bestowed parachute status on too many interventions. I can think of no better example for this analogy than treating hyperphosphatemia in CKD/ESRD. And it is this topic that would underlie my Dream RCT.
Imagining the Dream RCT in nephrology is an important exercise, but not for the reasons you might think. For many, there might be the temptation to devise trials that will pave the way for future enhancements to patient care or to some other exciting, forward thinking outcome. For me however, the Dream RCT in nephrology is about shattering or affirming nephrology dogma - and in nephrology we seem to have more dogma influencing core tenets of our specialty than in other medical disciplines. There is a popular saying among those in the clinical epidemiology world - that you don’t need to conduct an RCT to prove that parachutes are required when jumping out of a plane. Unfortunately in nephrology, thought leaders have bestowed parachute status on too many interventions. I can think of no better example for this analogy than treating hyperphosphatemia in CKD/ESRD. And it is this topic that would underlie my Dream RCT. Over the past 2 years, considerable excitement has been building over the results of the ACCOMPLISH study. This trial suggested that the combination of benazapril plus amlodipine is superior to benazapril plus hydrochlorothiazide for the prevention of a composite cardiovascular outcome. While there are methodological concerns regarding this trial that make me question its generalizability, it is thought-provoking to consider that one medication combination is superior to another even if blood-pressure between the 2 groups is negligible.
Over the past 2 years, considerable excitement has been building over the results of the ACCOMPLISH study. This trial suggested that the combination of benazapril plus amlodipine is superior to benazapril plus hydrochlorothiazide for the prevention of a composite cardiovascular outcome. While there are methodological concerns regarding this trial that make me question its generalizability, it is thought-provoking to consider that one medication combination is superior to another even if blood-pressure between the 2 groups is negligible. The TREAT Trial was presented at the ASN in San Diego. This much publicized trial examined the role of Aranesp in the management of diabetic patients with CKD. One group was randomized to receive Aranesp with a hemoglobin target of 130 g/l while the control group was given placebo and treated with Aranesp only if their hemoglobin fell below 90 g/l.
The TREAT Trial was presented at the ASN in San Diego. This much publicized trial examined the role of Aranesp in the management of diabetic patients with CKD. One group was randomized to receive Aranesp with a hemoglobin target of 130 g/l while the control group was given placebo and treated with Aranesp only if their hemoglobin fell below 90 g/l.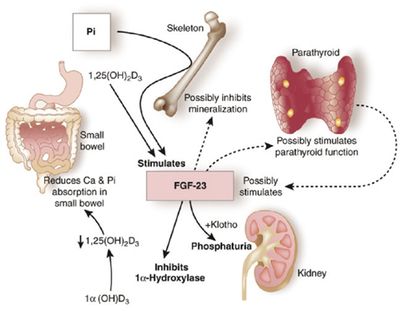 atemia has been been linked to poor patient outcomes, including a link to higher mortality. This relationship has been inferred by several retrospective and observational studies. In fact, the relationship between hyperphosphatemia and death is one of the most consistently espoused theories in all of nephrology. There is just problem however; there has never been a randomized trial to confirm this association.
atemia has been been linked to poor patient outcomes, including a link to higher mortality. This relationship has been inferred by several retrospective and observational studies. In fact, the relationship between hyperphosphatemia and death is one of the most consistently espoused theories in all of nephrology. There is just problem however; there has never been a randomized trial to confirm this association. In a fascinating article from November's
In a fascinating article from November's  Genome-wide association studies have previously shown a strong signal between a region residing on chromosome 22 centered on MYH9 and African Americans with FSGS and hypertension attributed end-stage kidney disease (H-ESKD). On July 15, 2010, Science released online a publication revealing a strong association between the same but expanded interval containing a nearby gene encoding for apolipoprotein L-1 (APOL1) in a similar group of patients.
Genome-wide association studies have previously shown a strong signal between a region residing on chromosome 22 centered on MYH9 and African Americans with FSGS and hypertension attributed end-stage kidney disease (H-ESKD). On July 15, 2010, Science released online a publication revealing a strong association between the same but expanded interval containing a nearby gene encoding for apolipoprotein L-1 (APOL1) in a similar group of patients. Here is a summary of the key findings:
Here is a summary of the key findings:
 This week in the New England Journal of Medicine, 2 studies reported on the use of mTor inhibitors in Autosomal Dominant Polycystic Kidney Disease (ADPKD). The results were mixed but overall, disappointing.
This week in the New England Journal of Medicine, 2 studies reported on the use of mTor inhibitors in Autosomal Dominant Polycystic Kidney Disease (ADPKD). The results were mixed but overall, disappointing.