As nephrologists with a significant interest in mineral metabolism practicing in Ontario, we are writing to inform you about a class review of all phosphate binders in end-stage renal disease that has been initiated by the Ontario Public Drug Program to evaluate and review their funding, as well as associated reimbursement criteria.
Controlling serum phosphorus, serum calcium and secondary hyperparathyroidism is a day-to-day challenge in the management of patients with end-stage renal disease on dialysis. Strategies to limit dietary phosphate intake and/or increasing the frequency or duration of dialysis when possible do not succeed in maintaining serum phosphate levels below 1.8 mmol/L and the majority of our patients require the use of oral phosphate binders in addition.
Calcium salts are the mainstay of pharmacological treatment, but many of our patients develop hypercalcemia and vascular calcification. The limitations associated with calcium salts have led to the development of newer non-calcium based agents, such as sevelamer and lanthanum, which have been widely adopted and funded worldwide. Other major Canadian provinces, including Quebec and British Columbia, also reimburse them. In Ontario, we have limited or no access to these drugs and we are concerned that this class review could result in further restrictions.
The recently published comprehensive evidence-based clinical practice guidelines1,2 stress the importance of maintaining serum phosphorus and calcium levels within an acceptable range. The Ontario Renal Network's (ORN) Clinical Advisory Committee has also established the percentage of dialysis patients who achieve a phosphate level of less than 1.8mmol/L as one of three patient outcome quality indicators for dialysis patients. http://www.renalnetwork.on.ca/quality/ Although the recent guidelines acknowledge the fact that there is limited evidence from randomized controlled clinical trials on the longer-term clinical outcomes, they support the need for a non-calcium based strategy in patients with high serum calcium levels.
While we acknowledge that the evidence from clinical trials to date does not entirely support the use of non-calcium based phosphate binders, we also believe that the federal and provincial committees responsible for recommending exclusion of these agents in the formularies have simultaneously ignored the evidence for harms arising from the use of calcium-based binders in the control subjects. Thus the need for access to non-calcium based phosphate binders should focus around issues of patient safety, and not simply those related to cost.
We would like to hear your opinions on this subject. We are also interested to learn whether terms of references have been established for this phosphate class review and whether the Ministry has involved the Ontario nephrology community, the Ontario Renal Network or the Ontario Association of Nephrologists
 Here is a summary of the key findings:
Here is a summary of the key findings: It is prevailing wisdom that patients with chronic kidney disease (CKD) progress more slowly if their blood pressure is well controlled. In fact, most modern guidelines suggest that for patients with CKD, a blood pressure of 130/80 should not be exceeded.
It is prevailing wisdom that patients with chronic kidney disease (CKD) progress more slowly if their blood pressure is well controlled. In fact, most modern guidelines suggest that for patients with CKD, a blood pressure of 130/80 should not be exceeded.
 Genome-wide association studies have previously shown a strong signal between a region residing on chromosome 22 centered on MYH9 and African Americans with FSGS and hypertension attributed end-stage kidney disease (H-ESKD). On July 15, 2010, Science released online a publication revealing a strong association between the same but expanded interval containing a nearby gene encoding for apolipoprotein L-1 (APOL1) in a similar group of patients.
Genome-wide association studies have previously shown a strong signal between a region residing on chromosome 22 centered on MYH9 and African Americans with FSGS and hypertension attributed end-stage kidney disease (H-ESKD). On July 15, 2010, Science released online a publication revealing a strong association between the same but expanded interval containing a nearby gene encoding for apolipoprotein L-1 (APOL1) in a similar group of patients.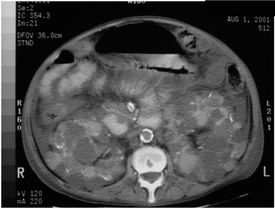 This week in the New England Journal of Medicine, 2 studies reported on the use of mTor inhibitors in Autosomal Dominant Polycystic Kidney Disease (ADPKD). The results were mixed but overall, disappointing.
This week in the New England Journal of Medicine, 2 studies reported on the use of mTor inhibitors in Autosomal Dominant Polycystic Kidney Disease (ADPKD). The results were mixed but overall, disappointing. In the
In the 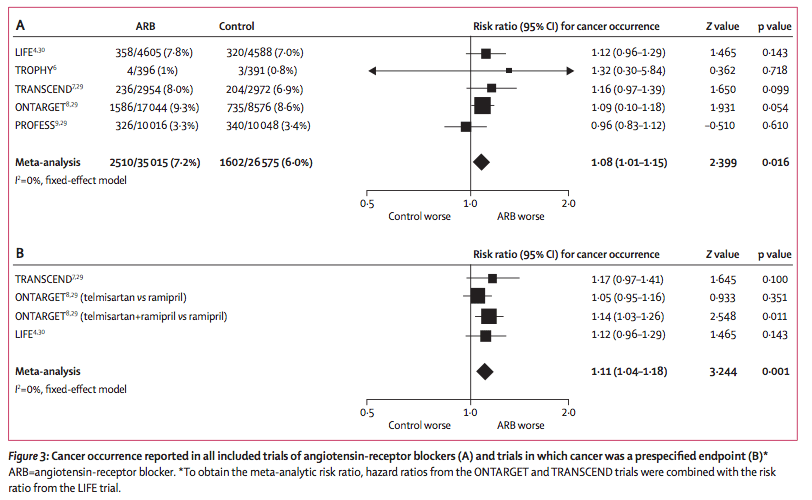
 I have never been a fan of vitamins. They fall in to a category of interventions with presumed safety and benefit. An important study in the
I have never been a fan of vitamins. They fall in to a category of interventions with presumed safety and benefit. An important study in the 